What is in this leaflet
This leaflet answers some common questions about Actiq. It does not contain all of the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of your taking Actiq against the benefits they expect it will have for you.
If you have any concerns about taking Actiq, talk to your doctor or pharmacist.
Keep this leaflet with the medicine. You may want to read it again.
What Actiq is used for
Actiq contains a medicine called fentanyl citrate. Fentanyl is a strong pain-relieving medicine and belongs to a group of medicine known as opioids.
Actiq lozenge with integral applicator is a system for delivering fentanyl directly through the lining of the mouth. When you place Actiq in your mouth, it dissolves and the medicine is absorbed through the lining of your mouth, into the blood system. Taking the medicine in this way allows it to be absorbed very quickly to relieve your breakthrough pain.
Breakthrough pain is additional sudden pain that occurs in spite of you having taken your usual opioid pain-relieving medicines.
Keep using the opioid pain medicine you take for your persistent (around-the-clock) cancer pain during your Actiq treatment.
Opioid medicines are those that contain active ingredients such as morphine, fentanyl citrate, codeine, methadone, oxycodone, pethidine or buprenorphine.
The use of Actiq by people who are not taking prescription opioid medicines on a regular basis could result in life-threatening side effects.
Ask your doctor if you have any questions about why Actiq lozenges have been prescribed for you. Your doctor may have prescribed it for another use.
Actiq is only available on a doctor's prescription.
Before you take it
Actiq is not suitable for everyone.
When you must not take it
Do not take Actiq if:
- you are allergic to fentanyl, or any of the other ingredients in the formulation (see list at the end of this leaflet)
- you have not been using a prescribed opioid pain medicine every day on a regular schedule, for at least one week, to control your persistent pain. This may increase the risk that your breathing could slow down to a dangerous level, or even stop
- you have short term pain, including pain from injuries, from doctors or dentists visits, surgery or headaches/migraines
- You are taking, or were taking in the last 2 weeks, a medicine belonging to a therapeutic class known as a monoamine-oxidase inhibitor.
- you have severe respiratory disease, severe obstructive lung conditions, acute respiratory disease and respiratory depression.
- You have never used an opioid pain medicine
- you are pregnant or breastfeeding, unless your doctor tells you it is safe.
Do not take Actiq after the expiry date shown on the blister package label and the carton.
The unit is normally white however, a slightly mottled appearance may occur on storage. This is due to slight changes in the flavouring of the product and does not affect how the product works in any way.
Do not take Actiq if the packaging seems to have been opened.
If you become pregnant whilst taking Actiq, you should stop taking it and see your doctor immediately. Young women taking Actiq and who are at risk of becoming pregnant should make sure that they are using adequate contraception.
Things to be careful of
Addiction
You can become addicted to Actiq even if you take it exactly as prescribed. Actiq may become habit forming causing mental and physical dependence. If abused it may become less able to reduce pain.
Dependence
As with all other opioid containing products, your body may becomeused to you taking Actiq. Taking it may result in physical dependence. Physical dependence means that you may experience withdrawal symptoms if you stop taking Actiq suddenly, so it is important to take it exactly as directed by your doctor.
Tolerance
Tolerance to Actiq may develop, which means that the effect of the medicine may decrease. If this happens, more may be needed to maintain the same effect.
Withdrawal
Continue taking your medicine for as long as your doctor tells you. If you stop having this medicine suddenly, your pain may worsen and you may experience some or all of the following withdrawal symptoms:
- nervousness, restlessness, agitation, trouble sleeping or anxiety
- body aches, weakness or stomach cramps
- loss of appetite, nausea, vomiting or diarrhoea
- increased heart rate, breathing rate or pupil size
- watery eyes, runny nose, chills or yawning
- increased sweating.
Actiq given to the mother during labour can cause breathing problems and signs of withdrawal in the newborn.
Diabetic patients should be advised that Actiq contains approximately 1.89 grams of sugar per unit.
Additionally, the risk of dental decay may be increased with frequent consumption of this medicine due to this sugar content.
Before you start to take it
Tell your doctor if:
- you have any allergies
- you are pregnant or intend to become pregnant
- you are breast feeding or planning to breast feed
- your other opioid pain medicine you take for your persistent (around-the-clock) cancer pain is not stabilized yet
- you have a history of addiction or substance abuse either personally or within your family
- you experience an unexplained increase in pain
- you notice any changes in hormones such as prolactin, testosterone and cortisol from blood tests
- you have or have had any of the following medical conditions:
- chronic diseases of the lung, or other condition which has an effect on your breathing
- head injury
- diabetes
- exceptionally slow heart rate
- liver or kidney disease.
These organs have an effect on the way in which your system breaks down the medicine.
Your doctor should monitor you for these signs while on treatment. They may also conduct blood tests from time to time to ensure you are responding as expected to treatment.
Taking other medicines
Tell your doctor if you are taking any other medicines, including any that you buy without a prescription from your pharmacy, supermarket or health food store.
Take special care with Actiq if:
- you are taking any medicines which might normally have a sedative effect (make you sleepy), such as:
- sleeping pills
- medicines to treat anxiety
- antihistamines
- tranquillisers. - you are taking any medicines or other substances that might have an effect on the way in which your body breaks down Actiq, such as:
- drugs used to treat fungal infections
- certain antibiotics
- grapefruit juice. - Antidepressants of the serotonergic type. Taking Actiq with these may increase the risk of serotonergic syndrome, a potentially life threatening condition
- muscle relaxants (such as cyclobenzaprine, metaxalone)
- you intend to drink alcohol, drive, or operate machinery in the few hours after taking Actiq. Your doctor will be able to advise you on this.
How to take it
Do not take Actiq to treat any condition other than that directed by your doctor.
How many to take
Your doctor will advise you on the appropriate starting dose of Actiq for the relief of your breakthrough pain.
Ask your doctor if you are not sure about the right dose or if you have any questions about taking Actiq. It is very important that you use this medicine in the manner as directed by your doctor.
You should start to feel some pain relief quickly while you are taking Actiq.
Until the dose that effectively controls your breakthrough pain has been determined, if you do not get enough pain relief from just one Actiq, your doctor may allow you to use a second dose to treat an episode of breakthrough pain.
Do not use a second Actiq unit unless your doctor tells you to do so and never use more than two Actiq units to treat a single episode of breakthrough pain. It is recommended that you wait at least 4 hours before treating another episode of breakthrough pain with Actiq.
You must tell your doctor immediately if you are using Actiq more than four times per day, as the doctor may wish to change your medicine for your persistent pain.
Once your persistent pain has been controlled, your doctor may need to change your dose of Actiq further.
For best results, let your doctor know about your pain and how Actiq is working for you so that the dose can be changed if needed.
Do not change doses of Actiq or your other pain medicines on your own.
Contact your doctor if your right dose of Actiq does not relieve your breakthrough pain. Your doctor will decide if your dose needs to be changed.
DO NOT TAKE MORE THAN THE DOSE YOUR DOCTOR HAS RECOMMENDED.
Change in dosage must be directed and monitored by your doctor.
How to take it
- Each Actiq unit is sealed in its own blister package. Do not open the package until you are ready to use it.
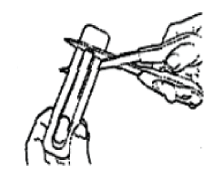
Hold the blister package with the printed side away from you. Grasp the short tab end of the blister package. Place scissors close to the end of the Actiq unit and cut the long tab end completely off (as shown). Separate the printed backing from the blister package and pull the printed backing completely off the blister package.
Remove the Actiq unit from the blister package.
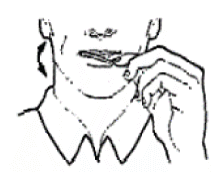
- Place Actiq in your mouth between your cheeks and gums. Using the handle, move it around in your mouth especially along your cheeks. Twirl the handle often. You may drink some water before using Actiq, to help with taking the product but you should not drink or eat anything while using Actiq.
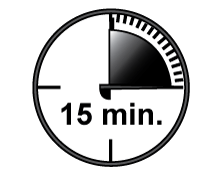
- To get the most effective relief, finish the Actiq completely in 15 minutes. If you finish it too quickly, you will swallow more of the medicine and get less relief of your breakthrough pain. It is also most important that you do not bite or chew Actiq for the same reason.
- If you begin to feel dizzy or sick before you have finished the medicine, remove the Actiq unit from your mouth and dispose of it immediately, as instructed in this leaflet. If for some reason you are not finishing the entire Actiq unit each time you have an episode of breakthrough pain, you should call your doctor.
- Once a dose has been arrived at which effectively controls your pain, you should not use more than four units of Actiq per day. If you think you might need to use more than four Actiq units per day, you should notify your doctor immediately.
- Do not use more than two Actiq units to treat a single episode of breakthrough pain.
Taking Actiq with food or drink
Actiq may be used before or after but not during meals. You may drink some water before using Actiq to help moisten your mouth, but you should not drink or eat anything while taking the medicine.
When to take it
Actiq must only be used for breakthrough pain. Breakthrough pain is additional sudden pain that occurs in spite of you having taken your usual opioid pain-relieving medicines.
How long to take it
You should not normally stop taking Actiq for breakthrough pain unless your doctor tells you to.
If you are taking high-doses of Actiq and feel that the pain is getting worse with time please speak to your doctor
Tell your doctor if you feel unwell during your course of treatment.
On the advice of your doctor, you may stop taking Actiq if you are no longer experiencing breakthrough pain. There are usually no noticeable effects if you stop taking Actiq. You should continue to take your usual opioid medicine to treat your persistent (around-the-clock) pain as instructed by your doctor.
How to dispose of Actiq after use
Partially used Actiq units may contain enough medicine to be harmful or life-threatening to a child.
Even if there is little or no medicine left on the handle, the handle itself must be properly disposed of, as follows:
- If the medicine is totally gone, throw the handle away in a waste container that is out of reach of children and pets.
- If any medicine remains on the handle, place the unit under hot running water to dissolve the remainder and then throw the handle away in a waste container that is out of reach of children and pets.
- If you do not finish the entire Actiq unit and you cannot immediately dissolve the remaining medicine, put the Actiq unit out of reach of children and pets until such a time as you can dispose of the partially used Actiq unit as instructed above.
Do not flush partially used Actiq units, Actiq handles, or the blister packaging down the toilet.
If you take too much (overdose)
If you or someone else receives too much (overdose), and experience one or more of the symptoms below, immediately call triple zero (000) for an ambulance. Keep the person awake by talking to them or gently shaking them every now and then. You should follow the above steps even if someone other than you have accidentally used Actiq that was prescribed for you. If someone takes an overdose, they may experience one or more of the following symptoms:
- Slow, unusual or difficult breathing
- Drowsiness, dizziness or unconsciousness
- Slow or weak heartbeat
- Nausea or vomiting
- Convulsions or fits
If you think you or someone else may have taken too much Actiq, you should immediately:
- telephone your doctor, or
- the Poisons Information Centre (telephone 13 11 26), or
- go to Accident and Emergency at your nearest hospital
Do this even if there are no signs of discomfort or poisoning.
When seeking medical attention, take this leaflet and remaining medicine with you to show the doctor. Also tell them about any other medicines or alcohol which have been taken.
The most common side effects are feeling sleepy, sick or dizzy. If you begin to feel very sleepy, remove the Actiq unit from your mouth and call another person to help you.
A serious side effect of Actiq is slow shallow breathing. This can occur if your dose of this medicine is too high or if you take too much Actiq. You and your carer should discuss this side effect with your doctor.
Note to carers
If you see that the patient taking Actiq has slow breathing or if you have a hard time waking the person up, take the following steps IMMEDIATELY:
- Using the handle, remove the Actiq unit from the person's mouth and keep it out of reach of children or pets until the Actiq unit is disposed of.
- CALL FOR EMERGENCY HELP.
- See instructions below for what to do if a child or adult accidentally takes Actiq.
What to do if a child or adult accidentally takes Actiq
If you think someone has accidentally taken Actiq follow these steps:
- If the person is asleep, wake them up by calling their name and shaking their arm or shoulder.
- CALL FOR EMERGENCY HELP.
- While waiting for emergency help:
- if the person seems to be breathing slowly, prompt them to breathe every 5-10 seconds
- if the person has stopped breathing give mouth-to-mouth resuscitation until help arrives.
If someone has accidentally taken Actiq, they may have the following symptoms:
- very sleepy
- itching, especially around the nose and eyes
- dizzy
- feeling sick or vomiting
- not breathing or breathing very slowly
While you are taking it
Things you must do
Make sure that all of your doctors and pharmacists know about your use of Actiq. Remind them if any new medicines are about to be started, including any medicines that you may purchase without a prescription.
Things you must not do
Do not use Actiq to treat any other complaints unless your doctor tells you to. It may not be safe to use Actiq for another complaint.
Do not give Actiq to someone else even if their symptoms are the same. Actiq should only be used by the person for whom it was prescribed. It may not be safe for another person to use Actiq.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking this medicine. Like all medicines, Actiq can cause side effects. Sometimes they are serious, most of the time they are not. You may need medical treatment if you get some of the side effects.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor if you notice any of the following and they worry you:
- headache
- nausea/vomiting
- feeling unwell
- weakness, dizziness
- sleepiness, sedation
- constipation
- anxiety or confusion
- anorexia
If you feel excessively dizzy, sleepy or otherwise ill while taking Actiq, use the handle to remove the Actiq unit and dispose of it according to the instructions given in this leaflet. Then contact your doctor for further directions on using Actiq.
Do not drive or operate machinery if you are feeling sleepy, dizzy or have difficulty in concentrating.
Tell your doctor immediately, or get someone to take you to Accident and Emergency at your nearest hospital if you notice any of the following:
- becoming very sleepy
- having slow or shallow breathing, shortness of breath
- sudden signs of allergy such as rash, itching or hives on the skin, swelling of the lips, tongue or throat, shortness of breath, wheezing or trouble breathing.
- if you have some or all of the following symptoms you may have something called serotonin syndrome: feeling confused, feeling restless, sweating, shaking, shivering, hallucinations, sudden jerks in your muscles or a fast heart beat
- vision abnormalities
- feeling faint or having trouble with speech
Whilst using the Actiq lozenge you may experience irritation, pain, gum bleeding or an ulcer at the site of application.
Tell your doctor if you notice anything else that is making you feel unwell. Other side effects not listed in this leaflet may also occur in some patients.
Do not be alarmed by this list of possible side effects. You may not experience any of them.
After using it
Storage
Keep Actiq lozenges in their individual blister package and carton box until it is time to take the dose. If you take Actiq out of its blister package, it may not keep as well.
Do not use it if the blister package has been damaged or opened before you are ready to use it.
Keep Actiq in a cool dry place where the temperature stays below 30°C.
Do not store it or any other medicine in the bathroom or near a sink. Do not leave it in the car on hot or cold days. Heat, cold and dampness can destroy some medicines.
Actiq lozenges must be kept out of the reach of children. The pain-relieving medicine in Actiq is very strong and could be life-threatening if taken accidentally by a child.
A locked cupboard at least one- and-a-half metres above the ground is a good place to store medicines.
Disposal
If you no longer need to use this medicine or it is out of date, take it to any pharmacy for safe disposal.
Product description
What Actiq looks like
The Actiq unit consists of a white to off-white solid medication attached to a handle (applicator).
Each unit contains either
200 micrograms,
400 micrograms,
600 micrograms,
800 micrograms,
1200 micrograms or 1600 micrograms of the active ingredient fentanyl.
The dosage strength is marked on the medication, on the handle and on the blister package to ensure that you are taking the right medicine.
Each blister package contains one individual Actiq unit.
Ingredients
Active ingredient:
The active ingredient is fentanyl, present in the product as fentanyl citrate.
Inactive ingredients:
Dextrates, citric acid, dibasic sodium phosphate, artificial berry flavour (maltodextrin, propylene glycol, artificial flavours, and triethyl citrate), magnesium stearate, confectioner's sugar (as sucrose and maize starch), purified water, and starch sodium octenyl succinate (E1450). The imprinting ink used contains de-waxed white shellac and brilliant blue FCF (CI42090).
Each unit of Actiq provides approximately 2 grams of glucose load from the dextrates.
Manufacturer/Distributor/Supplier
Actiq is supplied in Australia by:
Teva Pharma Australia Pty Ltd
Level 1, 37 Epping Rd.
Macquarie Park
NSW 2113
Telephone: 1800 28 8382
Australian Registration Numbers:
Actiq 200 micrograms:
AUST R 91598
Actiq 400 micrograms:
AUST R 91600
Actiq 600 micrograms:
AUST R 91601
Actiq 800 micrograms:
AUST R 91602
Actiq 1200 micrograms:
AUST R 91603
Actiq 1600 micrograms:
AUST R 91604.
ACTIQ is a registered trademark of Teva Pharmaceutical Industries Limited
This leaflet was revised in July 2021.
Published by MIMS September 2021

