AIMOVIG®
| Consumer Medicine Information (CMI) summary |
The full CMI on the next page has more details. If you are worried about using this medicine, speak to your doctor or pharmacist.
| ▼ This medicine is new or being used differently. Please report side effects. See the full CMI for further details. |
| 1. Why am I using Aimovig? |
Aimovig contains the active ingredient erenumab. Aimovig treats migraine. It is used to reduce the number of days each month you have a migraine. see Section 1. Why am I using Aimovig? in the full CMI.
| 2. What should I know before I use Aimovig? |
Do not use if you have had an allergic reaction to erenumab or any of the ingredients listed at the end of this leaflet.
Talk to your doctor if you have any other medical conditions, take any other medicines, are pregnant, plan to become pregnant or are breastfeeding. Aimovig is not suitable for children under 18 years. For more information, see Section 2. What should I know before I use Aimovig? in the full CMI
| 3. What if I am using other medicines? |
Some medicines may interfere with Aimovig and affect how it works. A list of these medicines is in Section 3. What if I am taking other medicines? in the full CMI.
| 4. How do I use Aimovig? |
The usual dose of Aimovig is 70 mg once every 4 weeks. Some patients may need to use 140 mg once every 4 weeks. Aimovig is supplied as a pen that contains a dose of 70 mg or 140 mg for self-administration. It is given as a single injection under the skin. Your doctor will train you or your care giver on how to use the Aimovig pen. Further instructions on how to use Aimovig can be found in Section 8. Instructions for use of Aimovig pre-filled pen? in the full CMI.
| 5. What should I know while using Aimovig? |
| Things you should do |
|
| Things you should not do |
|
| Driving or using machines |
|
| Looking after your medicine |
|
For more information, see Section 5. What should I know while using Aimovig? in the full CMI.
| 6. Are there any side effects? |
Common side effects include constipation, itching, muscle spasms, pain, redness and/or swelling at the injection site, hair loss, mouth or lip sores. Serious side effects are allergic reactions such as rash or swelling, or sometimes difficulty breathing.
For more information, including what to do if you have any side effects, see Section 6. Are there any side effects? in the full CMI.
| ▼ This medicine is subject to additional monitoring. This will allow quick identification of new safety information. You can help by reporting any side effects you may get. You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems. |
AIMOVIG® Solution for injection in a pre-filled pen.
Active ingredient(s): erenumab
| Consumer Medicine Information (CMI) |
This leaflet provides important information about using Aimovig.
You should also speak to your doctor or pharmacist if you would like further information or if you have any concerns or questions about using Aimovig.
Where to find information in this leaflet:
1. Why am I using Aimovig?
2. What should I know before I use Aimovig?
3. What if I am taking other medicines?
4. How do I use Aimovig?
5. What should I know while using Aimovig?
6. Are there any side effects?
7. Product details
8. Instructions for use of Aimovig pre-filled pen
| 1. Why am I using Aimovig? |
Aimovig contains the active ingredient erenumab. It belongs to a group of medicines called anti CGRP antibodies. CGRP stands for calcitonin gene related peptide.
Aimovig works by blocking the activity of the CGRP molecule, which has been linked to migraine.
Aimovig is used to treat migraine. It is intended to reduce the number of days each month you have a migraine.
| 2. What should I know before I use Aimovig? |
Warnings
Do not use Aimovig if:
- you are allergic to erenumab, or any of the ingredients listed at the end of this leaflet.
- Always check the ingredients to make sure you can use this medicine.
Allergic reactions can happen within minutes, but some may happen more than one week after using Aimovig.
Some of the symptoms of an allergic reaction may include:
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body
- rash, itching or hives on the skin.
Do not give this medicine to a child or adolescent under the age of 18 years as Aimovig has not been studied in this age group.
Do not use this medicine after the expiry date printed on the pack or if the packaging is torn or shows signs of tampering.
- If it has expired or is damaged, return it to your pharmacist for disposal.
Check with your doctor if you:
- have any other medical conditions for example:
- problems with your kidney
- problems with your liver
- high blood pressure
- history of constipation
- have had an allergic reaction to latex.
Aimovig contains sodium. The content is less than 1 mmol sodium (23 mg) per dose, this means it is essentially "sodium free" and should not affect a sodium-controlled diet.
If you have not told your doctor about any of the above, tell him/her before you start using Aimovig.
During treatment, you may be at risk of developing certain side effects. It is important you understand these risks and how to monitor for them. See additional information under Section 6. Are there any side effects?
Pregnancy and breastfeeding
Tell your doctor if are pregnant or plan to become pregnant.
Aimovig has not been studied in pregnant women. It is not known whether Aimovig could harm your unborn baby. Your doctor can discuss with you the risks and benefits involved.
- Tell your doctor if you are breastfeeding.
It is not known whether Aimovig passes into breast milk. Your doctor will help you decide whether you should stop breast feeding or stop taking Aimovig.
| 3. What if I am taking other medicines? |
Tell your doctor or pharmacist if you are taking any other medicines, including any medicines, vitamins or supplements that you buy without a prescription from your pharmacy, supermarket or health food shop.
Tell your doctor if you are taking other medicines for migraine.
Some medicines may interfere with Aimovig and affect how it works.
| 4. How do I use Aimovig? |
Follow all directions given to you by your doctor or pharmacist carefully.
They may differ from the information contained in this leaflet. If your doctor has prescribed Aimovig with another migraine medicine, follow your doctor's instructions on how to use these medicines together.
If you do not understand the instructions in the box, ask your doctor or pharmacist for help.
How much and when to use Aimovig
Each pen contains 70 mg or 140 mg Aimovig. The usual dose of Aimovig is 70 mg once every 4 weeks. Your doctor might decide you need 140 mg once every 4 weeks. Use Aimovig exactly as instructed by your doctor.
70 mg/mL solution for injection
If your doctor prescribed the 70 mg dose you should use one 70 mg/mL pen once every 4 weeks, injected under the skin in your abdomen, thigh or upper arm.
However, if your doctor prescribed the 140 mg dose you can use two 70 mg pens once every 4 weeks. The second injection must be given immediately after the first.
140 mg/mL solution for injection
If your doctor prescribed the 140 mg dose, you will use one 140 mg/mL pen once every 4 weeks, injected under the skin in your abdomen, thigh, or upper arm.
Make sure that you inject the entire contents of each pen.
How to use Aimovig
Your doctor or nurse will give you or your caregiver training in the right way to prepare and inject Aimovig. Do not try to inject Aimovig until you have completed this training.
Instructions for how to use Aimovig are provided under section 8. Instructions for use of Aimovig pre-filled pens?
If you forget to use Aimovig
Aimovig should be used regularly on the same day each month. If you miss your dose at the usual time, use it as soon as possible. Your next dose is then taken one month from the date of your last dose.
Contact your doctor or pharmacist if you are not sure when to take your next dose.
Do not use a double dose to make up for the dose that you missed.
If you use too much Aimovig
If you think that you have used too much Aimovig, you may need medical attention.
You should immediately:
- phone the Poisons Information Centre
(by calling 13 11 26), or - contact your doctor, or
- go to the Emergency Department at your nearest hospital.
You should do this even if there are no signs of discomfort or poisoning.
| 5. What should I know while using Aimovig? |
Things you should do
After 8 to 12 weeks of starting Aimovig visit your doctor who will assess your progress. Some effects of Aimovig such as high blood pressure can only be checked by a doctor.
Schedule a visit with your doctor every 3 to 6 months to check your progress.
If you are about to be started on any new medicine, remind your doctor and pharmacist that you are using Aimovig.
Tell any other doctors, dentists, and pharmacists who treat you that you are using this Aimovig.
If you become pregnant while taking this medicine, tell your doctor immediately.
Things you should not do
Do not use Aimovig to treat any other complaints unless your doctor tells you to.
Do not give your medicine to anyone else, even if they have the same condition as you.
Do not stop using your medicine or lower the dosage without checking with your doctor. Your symptoms may return if you stop the treatment.
Driving or using machines
Aimovig is not expected to affect driving or ability to use machinery.
Drinking alcohol
Tell your doctor if you drink alcohol regularly.
Looking after your medicine
Follow the instructions in the carton on how to take care of your medicine properly.
- Keep the pen in the outer carton until it is time to use it. If you take the pen out of the carton it may not keep well.
- Keep pens in a refrigerator (2°C - 8°C). Do not freeze. Do not shake.
- After Aimovig has been taken out of the refrigerator, it can be kept at room temperature (up to 30°C) in the outer carton box and must be used within 7 days. Do not put Aimovig back in the refrigerator after it has reached room temperature.
Store it in a cool dry place away from moisture, heat or sunlight; for example, do not store it:
- in the bathroom or near a sink, or
- in the car or on window sills.
Keep it where young children cannot reach it.
- A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
When to discard your medicine
Aimovig pen is for single use only.
If your doctor tells you to stop using this medicine or the expiry date has passed, ask your pharmacist what to do with any medicine that is left over.
Do not use this medicine after the expiry date which is stated on the label and carton after EXP. The expiry date refers to the last day of that month.
Getting rid of any unwanted medicine
If you no longer need to use this medicine or it is out of date, take it to any pharmacy for safe disposal.
Do not throw away any medicines via wastewater or household waste.
| 6. Are there any side effects? |
All medicines can have side effects. If you do experience any side effects, most of them are minor and temporary. However, some side effects may need medical attention.
See the information below and, if you need to, ask your doctor or pharmacist if you have any further questions about side effects.
Less serious side effects
| Less serious side effects | What to do |
| Speak to your doctor if you have any of these less serious side effects and they worry you. |
Serious side effects
| Serious side effects | What to do |
Allergic reactions
| Call your doctor straight away or go straight to the Emergency Department at your nearest hospital if you notice any of these serious side effects. |
Tell your doctor or pharmacist if you notice anything else that may be making you feel unwell.
Other side effects not listed here may occur in some people. Some side effects may not give you any symptoms and can only be found when tests are done. For example, the development or worsening of high blood pressure.
Reporting side effects
After you have received medical advice for any side effects you experience, you can report side effects to the Therapeutic Goods Administration online at www.tga.gov.au/reporting-problems. By reporting side effects, you can help provide more information on the safety of this medicine.
Always make sure you speak to your doctor or pharmacist before you decide to stop using any of your medicines.
| 7. Product details |
This medicine is only available with a doctor's prescription.
What Aimovig contains
| Active ingredient (main ingredient) | Erenumab |
| Other ingredients(inactive ingredients) | Sucrose Polysorbate 80 Sodium hydroxide Glacial acetic acid Water for injections. |
| Potential allergens | Natural rubber latex is included within the needle cap. |
| Other information | This medicine does not contain lactose, gluten, tartrazine or any other azo dyes. |
Do not take this medicine if you are allergic to any of these ingredients.
What Aimovig looks like
Aimovig is a solution which is clear to opalescent, colourless to light yellow, and practically free from particles. Do not use the solution if you notice that it contains easily visible particles, is cloudy or is distinctly yellow.
Aimovig 70 mg solution for injection in pre-filled pen: AUST R 289959.
Aimovig 140 mg solution for injection in pre-filled pen: AUST R 309205.
Not all presentations are available.
Who distributes Aimovig?
Novartis Pharmaceuticals Australia Pty Limited
ABN 18 004 244 160
54 Waterloo Road
Macquarie Park NSW 2113
Telephone 1 800 671 203
Web site: www.novartis.com.au
This leaflet was prepared in October 2022.
® Registered trademark. © Copyright 2022.
(aimpen041022c.doc based on PI aim041022i.doc)
| 8. Instructions for use of Aimovig pre-filled pen |
See also www.migrainedirect.com.au, where there is a link to a video showing how to use the Aimovig pen.
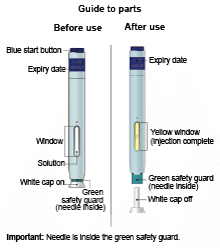
Single-Use Autoinjector/Pen 70 mg/mL
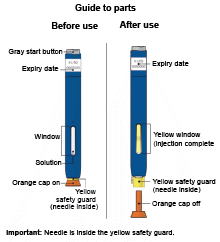
Single-Use Autoinjector/Pen 140 mg/mL

Important: read before use
Storing your Aimovig pre-filled pen
- Keep the pen out of the reach of children.
- Keep the pen in the outer carton in order to protect from light.
- Store in a refrigerator (2°C to 8°C). Do not freeze.
- Throw away any pen that has been left at room temperature (up to 30°C) for more than 7 days.
- Do not store the pen in extreme heat or cold. For example, avoid storing in your car.
Things not to do
- Do not try to inject Aimovig before receiving training from the doctor or nurse.
- Do not use this pen of the expiry date stated on the label has passed.
- Do not shake the pen.
- Do not remove the white or orange cap from the pen until you are ready to inject.
- Do not use the pen if it has been dropped on a hard surface. This is because part of the pen may be broken even if you cannot see the break. Use a new pen and contact your doctor or pharmacist.
Step 1: Prepare
Your healthcare provider has prescribed a 70 mg or 140 mg dose.
For a 70 mg dose, inject one pen of 70 mg/mL.
For a 140 mg dose, inject either two pens of 70 mg/mL one after the other, or one pen of 140 mg/mL as a single injection.
A) Carefully lift the pen(s) out of the carton
To avoid discomfort at the site of injection, leave the pen(s) at room temperature for at least 30 minutes before injecting.
Important
- Do not put the pen(s) back in the refrigerator once they have reached room temperature.
- Do not try to warm the pen(s) by using a heat source such as hot water or microwave.
- Do not leave the pen(s) in direct sunlight.
- Do not shake the pen(s).
- Do not remove the white or orange cap from the pen(s) at this stage.
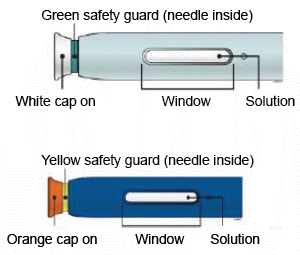
B) Inspect each pen
Make sure the solution you see in the window is clear and colourless to light yellow.
Important
- Do not use the pen if you notice that the solution contains easily visible particles, is cloudy or is distinctly yellow.
- Do not use the pen if any part of it appears cracked or broken.
- Do not use the pen if it has been dropped.
- Do not use the pen if white or orange cap is missing or is not securely attached.
- Do not use the pen after the expiry date stated on the label.
In all cases described above, use a new pen, and if you are unsure contact your doctor or pharmacist.
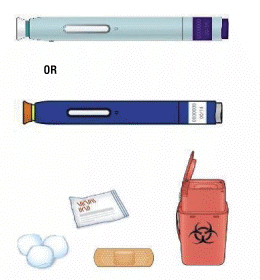
C) Gather all materials needed for the injection(s):
Wash your hands thoroughly with soap and water.
On a clean, well-lit work surface, place the:
- One or two new pen(s)
- Alcohol wipes
- Cotton balls or gauze pads
- Adhesive plasters
- Sharps disposal container.
D) Prepare and clean the injection site(s).
You can use any of the following injection sites:
- Thigh
- Stomach area (abdomen) (except for a 5 cm area around the navel)
- Outer area of upper arm (only if someone else is giving you the injection).
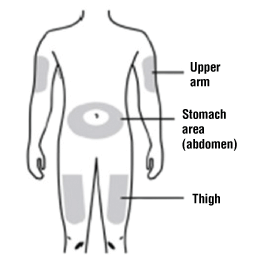
Clean the injection site with an alcohol wipe and let the skin dry.
Choose a different site each time you give yourself an injection. If you need to use the same injection site, just make sure it is not the same spot on that site you used last time.
Important
After you have cleaned the area, do not touch it again before injecting.
Do not choose an area where the skin is tender, bruised, red, or hard. Avoid injecting into a raised, thick, red or scaly skin patch or lesion, or areas with scars or stretch marks.
Step 2: Get ready
E) Pull the white or orange cap straight off, only when you are ready to inject. The injection must be administered within 5 minutes. It is normal to see a drop of liquid at the end of the needle or green or yellow safety guard.
Important
- Do not remove the white or orange cap from the pen until you are ready to inject.
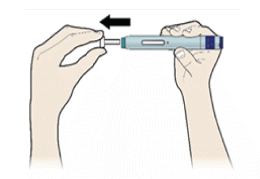
- Do not leave the white or orange cap off for more than 5 minutes. This can dry out the medicine.
- Do not twist or bend the white/orange cap.
- Do not put the white or orange cap back onto the pen once it has been removed.
F) Stretch or pinch the injection site to create a firm surface.
Stretch method
Stretch skin firmly by moving your thumb and fingers in opposite directions, creating an area about five cm wide.
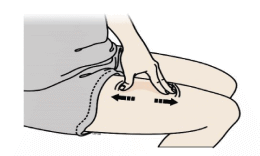
Or
Pinch method
Pinch skin firmly between your thumb and fingers, creating an area about five cm wide.
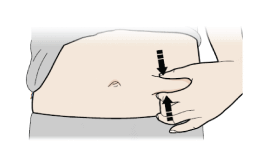
Important
Keep skin stretched or pinched while injecting.
Step 3: Inject
G) Keep holding the stretch or pinch. With the white or orange cap off, place the pen on the skin at an angle of 90 degrees.
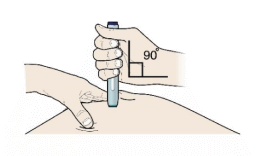
Important
Do not touch the purple or grey start button yet.
H) Firmly push the pen down onto the skin until it stops moving.
Push down
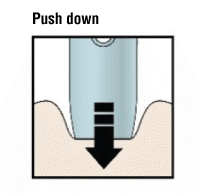
Important: You must push all the way down but do not touch the purple or grey start button until you are ready to inject.
I) When you are ready to inject, press the purple or grey start button.
You will hear a click.
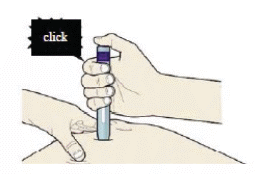
J) Keep pushing down on the skin. The injection could take about 15 seconds.
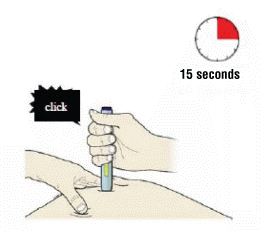
Important: Window turns yellow when injection is completed.
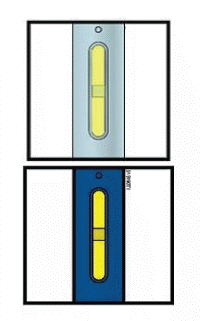
Note: After you remove the pen from the skin, the needle will automatically be covered by the green or yellow safety guard.
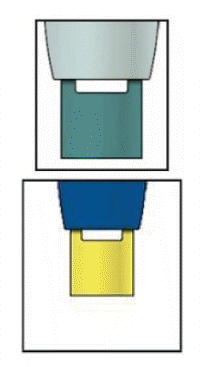
Important: When you remove the pen, if the window has not turned yellow, or if it looks like the medicine is still injecting, this means you have not received a full dose.
Contact your doctor immediately.
Step 4: Finish
K) Discard the used pen and the white or orange cap.
Put the used pen in a sharps disposal container immediately after use. Talk to your doctor or pharmacist about proper disposal. There may be local regulations for disposal.
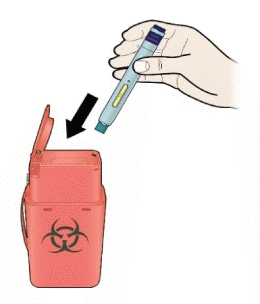
Important
- Do not reuse the pen.
- Do not recycle the pen or sharps disposal container, or throw them into household waste.
- Always keep the sharps disposal container out of the sight and reach of children.
L) Examine the injection site.
If there is a small sign of blood, press a cotton ball or gauze pad onto the injection site. Do not rub the injection site. Apply an adhesive plaster if needed.

If you have been prescribed a 140 mg dose using two 70 mg/mL pens, a second injection is required. Repeat all steps with the second pen to inject the full 140 mg dose.
Commonly asked questions
- What will happen if I press the purple or grey start button before I am ready to inject?
Even if you press the purple or grey start button, the injection will only happen when the green or yellow safety guard is also pushed into the pen.
- Can I move the pen around on my skin while I am choosing an injection site?
You can move the pen around on the injection site provided you do not press the purple or grey start button. However, if you press the purple or grey start button and the green or yellow safety guard is pushed into the pen, the injection will begin.
- Can I release the purple or grey start button after I start the injection?
You can release the purple or grey start button, but keep holding the pen firmly against the skin during the injection.
- Will the purple or grey start button pop up after I release my thumb?
The purple or grey start button may not pop up after you release your thumb if you held your thumb down during the injection. This is not a problem.
- What do I do if I didn't hear a click after pushing the pen down on my skin for 15 seconds?
If you didn't hear a click, you can check that a complete injection was given by looking whether the window has turned yellow.
- Who do I contact if I need help with the pen or with the injection?
If you have any questions about the pen or its storage, or about the injection, contact your doctor or pharmacist.
For the most current information about this product, ask your pharmacist or doctor for a copy of the latest Consumer Medicine Information (CMI), or go to www.novartis.com.au (Australia) or www.medsafe.govt.nz (New Zealand).
Aimovig is distributed by:
Novartis Pharmaceuticals Australia Pty Limited
ABN 18 004 244 160
54 Waterloo Road
Macquarie Park NSW 2113
Telephone 1 800 671 203
Web site: www.novartis.com.au
Novartis New Zealand Limited
PO Box 99102
Newmarket
Auckland 1149
Telephone: 0800 354 335
Date of Preparation: October 2022
(aimpen041022l.doc)
Published by MIMS November 2022
