What is in this leaflet
Please read this leaflet carefully before you start taking AMBRISENTAN VIATRIS.
This leaflet answers some common questions about AMBRISENTAN VIATRIS.
It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have benefits and risks. Your doctor has weighed the risks of you taking AMBRISENTAN VIATRIS against the benefits expected for you.
If you have any concerns about taking this medicine, talk to your doctor or pharmacist.
Keep this leaflet with your medicine. You may need to read it again.
What AMBRISENTAN VIATRIS is used for
AMBRISENTAN VIATRIS contains the active ingredient ambrisentan which is a type of medicine called an endothelin receptor antagonist (ERA). It is used to treat adults with pulmonary arterial hypertension (PAH), which is high blood pressure in the blood vessels (the pulmonary arteries) that carry blood from the heart to the lungs. In people with PAH, these arteries get narrower, so the heart has to work harder to pump blood through them. This causes people to become tired, dizzy and short of breath.
This medicine works by widening the pulmonary arteries, making it easier for your heart to pump blood through them. This lowers the blood pressure and relieves your symptoms.
AMBRISENTAN VIATRIS is not recommended for use in children and patients under 18 years of age as there have been no studies of its effects in this age group.
AMBRISENTAN VIATRIS must not be used for the treatment of idiopathic pulmonary fibrosis (IPF) and must not be used in patients who have IPF with or without secondary pulmonary hypertension.
AMBRISENTAN VIATRIS is available as 5 mg and 10 mg tablets.
This medicine is available only with a doctor's prescription
Before you take AMBRISENTAN VIATRIS
When you must not take it
Do not take AMBRISENTAN VIATRIS if you have an allergy to:
- any medicine containing ambrisentan
- any of the ingredients listed at the end of this leaflet
- any other similar medicines.
Some of the symptoms of an allergic reaction may include:
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body
- rash, itching or hives on the skin
Do not take AMBRISENTAN VIATRIS if you are pregnant or planning to become pregnant. It may affect your developing baby if you take it during pregnancy or if you become pregnant soon after you stop treatment.
If it is possible you could become pregnant, you must use reliable birth control (contraception) while using AMBRISENTAN VIATRIS and for three months after you stop taking it.
Use at least two reliable forms of birth control (contraception) while you are taking AMBRISENTAN VIATRIS. Talk to your doctor about this.
If you are a woman who could become pregnant, your doctor will ask you to take a pregnancy test before you take AMBRISENTAN VIATRIS.
If you are a male you should avoid exposing your partner to your semen by use of appropriate contraception e.g. condoms.
If you do become pregnant, talk to your doctor immediately.
Do not take AMBRISENTAN VIATRIS if:
- you have scarring of the lungs of unknown cause (a condition known as idiopathic pulmonary fibrosis or IPF)
- you have or have had a serious liver problem
- you have raised levels of some liver enzymes (detected by blood tests).
Do not take this medicine after the expiry date printed on the pack or if the packaging is torn or shows signs of tampering. If it has expired or is damaged, return it to your pharmacist for disposal.
If you are not sure whether you should start taking this medicine, talk to your doctor.
Before you start to take it
Tell your doctor if you have allergies to any other medicines, foods, preservatives or dyes.
Tell your doctor if you are breastfeeding or plan to breastfeed Your doctor can discuss with you the risks and benefits involved.
Tell your doctor if you have or have had any of the following medical conditions:
- liver disease
- heart disease called right heart failure
- a low number of red blood cells (anaemia)
- if you have swelling, especially of the ankles and feet (oedema)
- low blood pressure
- kidney problems.
If any of these applies to you, your doctor will decide if AMBRISENTAN VIATRIS is suitable for you.
Blood tests
Your doctor will take blood tests to check:
- whether you have a reduced number of red blood cells (anaemia)
- that your liver is working properly.
These blood tests will be taken before you start taking AMBRISENTAN VIATRIS and throughout your treatment with this medicine.
Taking other medicines
Tell your doctor or pharmacist if you are taking any other medicines, including any that you get without a prescription from a pharmacy, supermarket or health food shop.
Some medicines and AMBRISENTAN VIATRIS may interfere with each other. These include:
- cyclosporin A (a medicine taken if you have an organ transplant). If you take cyclosporin A, do not take more than one 5 mg tablet of AMBRISENTAN VIATRIS, once a day
- ketoconazole (a medicine used to treat fungus infection).
These medicines may be affected by AMBRISENTAN VIATRIS or may affect how well it works. You may need different amounts of your medicines, or you may need to take different medicines.
Your doctor and pharmacist have more information on medicines to be careful with or avoid while taking this medicine.
How to take AMBRISENTAN VIATRIS
Follow all directions given to you by your doctor and pharmacist carefully. They may differ from the information contained in this leaflet.
If you do not understand the instructions, ask your doctor or pharmacist for help.
How much to take
The usual dose of AMBRISENTAN VIATRIS is 5 mg, once a day. Your doctor may decide to increase your dose to 10 mg, once a day.
If you take cyclosporin A, do not take more than one 5 mg tablet of AMBRISENTAN VIATRIS, once a day.
How to take it
Swallow the tablets whole with a full glass of water.
AMBRISENTAN VIATRIS tablets should not be split, crushed or chewed.
You can take this medicine with or without food.
When to take it
Take your medicine at about the same time each day. Taking it at the same time each day will have the best effect. It will also help you remember when to take it.
It does not matter if you take this medicine before or after food.
How long to take it
Continue taking your medicine for as long as your doctor tells you to.
This medicine helps to control your condition, but does not cure it. It is important to keep taking your medicine even if you feel well.
If you forget to take it
Take it as soon as you remember, and then go back to taking your medicine as you would normally.
Do not take a double dose to make up for the dose you missed. This may increase the chance of you getting an unwanted side effect.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering to take your medicine, ask your pharmacist for some hints.
If you take too much (overdose)
Immediately telephone your doctor or the Poisons Information Centre (telephone 13 11 26) for advice, or go to Accident and Emergency at the nearest hospital, if you think that you or anyone else may have taken too much AMBRISENTAN VIATRIS. Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
While you are taking AMBRISENTAN VIATRIS
Things you must do
If you are about to be started on any new medicine, remind your doctor and pharmacist that you are taking AMBRISENTAN VIATRIS.
Tell any other doctors, dentists and pharmacists who treat you that you are taking this medicine.
You must use reliable birth control (contraception) while using AMBRISENTAN VIATRIS and for 3 months after you stop taking it. Use at least two reliable forms of birth control (contraception).
If you become pregnant while taking this medicine, tell your doctor immediately.
If you are a male, you should avoid exposing your partner to your semen by use of appropriate contraception e.g. condoms.
Keep all of your doctor's appointments so that your progress can be checked. Your doctor will need to take regular blood tests while you are taking this medicine to ensure your liver function and red blood cell (anaemia) levels remain normal and to prevent unwanted side effects.
Things you must not do
Do not take AMBRISENTAN VIATRIS to treat any other complaints unless your doctor tells you to.
Do not give your medicine to anyone else, even if they have the same condition as you.
Do not stop taking your medicine or change the dosage without checking with your doctor.
Things to be careful of
It is not known whether AMBRISENTAN VIATRIS affects your ability to drive or use machines. However, the symptoms of your condition can make you less fit to drive.
Do not drive or operate machines if you are feeling unwell.
If you feel light-headed, dizzy or faint when getting out of bed or standing up, get up slowly. Standing up slowly, especially when you get up from bed or chairs, will help your body get used to the change in position and blood pressure. If this problem continues or gets worse, talk to your doctor.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking AMBRISENTAN VIATRIS.
This medicine helps most people with pulmonary arterial hypertension, but it may have unwanted side effects in a few people.
All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical attention if you get some of the side effects.
If you are over 65 years of age you may have an increased chance of getting side effects.
Do not be alarmed by the following list of side effects. You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
Very common side effects (could affect more than one in ten people) include:
- swelling (oedema), especially in the ankles and feet
- headache
- a runny or blocked nose, congestion or pain in the sinuses
- dizziness
- palpitations (fast or irregular heartbeat)
- feeling tired, lack of energy (fatigue)
- worsening shortness of breath shortly after starting AMBRISENTAN VIATRIS
- signs of anaemia, such as tiredness, weakness, shortness of breath and feeling generally unwell
Other common side effects (could affect up to one in ten people) include:
- constipation
- pain in your stomach
- feeling weak
- vomiting
- heart failure
- hypotension
- fainting
- abnormal blood tests for liver function
- runny nose
- chest pain or discomfort
- flushing
- nose bleed
- rash
- allergic reactions: you may notice a rash or itching or swelling (usually of the face, lips or throat), which may cause difficulty in breathing or swallowing
- visual disturbance (blurred vision or changes in your ability to see clearly).
Uncommon side effects include:
- signs that your liver may not be working as it normally should, including:
- loss of appetite
- sick (nausea)
- vomiting
- fever
- unusual tiredness
- pain in the stomach (abdominal pain)
- yellow colouring of your skin or the whites of your eyes (jaundice)
- your urine turns dark in colour
- itching of the skin
- abnormal liver function which may show up in your blood tests
If you notice any of these signs, tell your doctor immediately.
Tell your doctor or pharmacist if you notice anything that is making you feel unwell.
Other side effects not listed above may also occur in some people.
After taking AMBRISENTAN VIATRIS
Storage
Keep your tablets in the pack until it is time to take them. If you take the tablets out of the pack they may not keep well.
Keep your tablets in a cool dry place where the temperature stays below 25°C.
Do not store AMBRISENTAN VIATRIS or any other medicine in the bathroom or near a sink. Do not leave it on a window sill or in the car. Heat and dampness can destroy some medicines.
Keep it where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking this medicine or the expiry date has passed, ask your pharmacist what to do with any medicine that is left over.
Product description
What it looks like
AMBRISENTAN VIATRIS is presented in packs of 30 tablets in two available strengths of 5 mg and 10 mg.
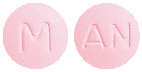
The 5 mg tablets are pink, film-coated, round, biconvex tablets debossed with ‘M’ on one side of the tablet and ‘AN’ on the other side.
The 10 mg tablets are pink, film-coated, capsule shaped, biconvex tablets debossed with ‘M’ on one side of the tablet and ‘AN1’ on the other side.
Ingredients
AMBRISENTAN VIATRIS contains 5 mg and 10 mg of ambrisentan as the active ingredient.
It also contains the following inactive ingredients:
- Microcrystalline cellulose
- Lactose
- Croscarmellose sodium
- Magnesium stearate
- OPADRY II complete film coating system 85F540046 PINK (ARTG PI No:107526).
AMBRISENTAN VIATRIS contains lactose.
Supplier
AMBRISENTAN VIATRIS is supplied in Australia by:
Alphapharm Pty Ltd trading as Viatris
Level 1, 30 The Bond
30-34 Hickson Road
Millers Point NSW 2000
www.viatris.com.au
Phone: 1800 274 276
Australian registration numbers:
AMBRISENTAN VIATRIS 5 mg:
AUST R 298940
AMBRISENTAN VIATRIS 10 mg:
AUST R 298941
This leaflet was prepared in September 2022.
AMBRISENTAN VIATRIS_cmi \Sep22/00
Published by MIMS October 2022