1 Name of Medicine
Sertraline hydrochloride.
2 Qualitative and Quantitative Composition
Each tablet contains 50 mg or 100 mg sertraline (as sertraline hydrochloride).
For the full list of excipients, see Section 6.1 List of Excipients.
3 Pharmaceutical Form
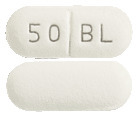
50 mg tablets. White to off white, capsule shaped, biconvex, film coated tablets with breakline on one side and '50' and 'BL' debossed on either side of the breakline.
100 mg tablets. White to off white, capsule shaped, biconvex, film coated tablets with '100' and 'BL' debossed on one side.
4 Clinical Particulars
4.9 Overdose
On the evidence available, sertraline has a wide margin of safety in overdose. Overdoses in adults of 700 to 2100 mg have not resulted in serious symptoms. Ingestion of 4000 mg resulted in seizures in an adolescent. The largest known ingestion is 13.5 g with recovery reported. Another overdose of 2.5 g of sertraline alone resulted in death. Overdosage of 400 and 500 mg in two children have resulted in serotonin syndrome.
Symptoms. Symptoms of overdose include serotonin mediated side effects such as electrocardiogram QT prolonged, torsades de pointes (see Section 4.4 Special Warnings and Precautions for Use; Section 4.5 Interactions with Other Medicines and Other Forms of Interactions; Section 5.1 Pharmacodynamic Properties, Clinical trials), somnolence, gastrointestinal disturbances (such as nausea, diarrhoea and vomiting), tachycardia, tremor, agitation and dizziness. Other important adverse events reported with sertraline overdose (single or multiple drugs) include bradycardia, bundle branch block, coma, convulsions, delirium, hallucinations, hypertension, hypotension, manic reaction, pancreatitis, QT-interval prolongation, stupor and syncope. Hyperthermia, increased respirations and cutaneous vasodilation have also been reported. Minor ECG abnormalities, palpitations, prolonged tachycardia and increased pulse rate have also been reported following paediatric overdose. Seizures have been reported rarely. Serotonin syndrome may result following significant overdose, and onset may be delayed. A death due to asthma exacerbation has been reported following sertraline overdose.
Deaths have been reported involving overdoses of sertraline, primarily in combination with other drugs and/or alcohol. Therefore any overdosage should be treated aggressively.
Elevated liver enzymes and elevated creatine phosphokinase levels have been noted following acute overdose. Hyponatraemia secondary to SIADH has been reported following overdose and has been severe enough to cause seizures.
In managing overdosage, consider the possibility of multiple drug involvement. Treatment should consist of those general measures employed in the management of overdosage with any antidepressant. Cardiac and vital signs monitoring is recommended along with general symptomatic and supportive measures. Establish and maintain an airway, ensure adequate oxygenation and ventilation, if necessary. Patients should be monitored for potential cardiovascular, gastrointestinal or hepatic abnormalities. Also monitor for signs/ symptoms of serotonin syndrome (mental status changes, hyperthermia, myoclonus, autonomic instability, high CK levels) and possible seizures.
Treatment. There are no specific antidotes for sertraline. Activated charcoal should be considered in treating overdose and is most effective when administered within one hour of ingestion. In patients who are not fully conscious or have impaired gag reflex, consideration should be given to administering activated charcoal via nasogastric tube once the airway is protected. Routine use of a cathartic with activated charcoal is not recommended as there is no evidence that cathartics reduce drug absorption and cathartics are known to cause adverse effects such as nausea, vomiting, abdominal cramps, electrolyte imbalances and occasionally hypotension.
Induction of emesis is not recommended because of the potential for CNS depression and seizures. Due to the large volume of distribution of sertraline, forced diuresis, dialysis, haemoperfusion, and exchange transfusion are unlikely to be of benefit.
For information on the management of overdose, contact the Poison Information Centre on 131126 (Australia).
5 Pharmacological Properties
5.3 Preclinical Safety Data
Genotoxicity. Sertraline had no genotoxic effects, with or without metabolic activation, based on the following assays; bacterial mutation assay; mouse lymphoma mutation assay; and tests for cytogenetic aberrations in vivo in mouse bone marrow and in vitro in human lymphocytes.
Carcinogenicity. The carcinogenic potential of sertraline has not been fully elucidated. Lifetime carcinogenicity studies were carried out in CD-1 mice and Long-Evans rats (at doses up to 40 mg/kg), giving rise to plasma drug exposure levels similar to or slightly higher than that achieved following the maximum recommended human dose of 200 mg. There was a dose related increase in the incidence of liver adenomas in male mice receiving sertraline at 10 mg/kg to 40 mg/kg. No increase was seen in female mice or in rats of either sex receiving the same treatments, nor was there an increase in hepatocellular carcinomas. Liver adenomas have a variable rate of spontaneous occurrence in the CD-1 mouse and are of unknown significance to humans. There was an increase in follicular adenomas of the thyroid in female rats receiving sertraline at 40 mg/kg; this was not accompanied by thyroid hyperplasia. While there was an increase in uterine adenocarcinomas in rats receiving sertraline at 10-40 mg/kg compared to placebo controls, this effect was not clearly drug related.
6 Pharmaceutical Particulars
6.7 Physicochemical Properties
Sertraline hydrochloride is a white to off-white crystalline powder that is slightly soluble in water and isopropyl alcohol and sparingly soluble in ethanol and dimethylformamide.
Sertraline hydrochloride is a selective serotonin re-uptake inhibitor (SSRI) antidepressant for oral administration. It is chemically unrelated to tricyclic, tetracyclic or other available antidepressant agents.
Sertraline is a disubstituted tetrahydronaphthalene with two asymmetric centres and can exist as four enantiomeric forms in either the trans- or cis-configuration. The cis-(1S,4S) sertraline enantiomer is used.
Chemical structure. Structural formula:
https://stagingapi.mims.com/au/public/v2/images/fullchemgif/CSSERHYD.gif Chemical name: (1S,4S)-4-(3,4-dichlorophenyl)-1,2,3,4-tetrahydro-N-methyl-1-naphthalenamine hydrochloride.
Molecular formula: C17H17Cl2N.HCl.
Molecular weight: 342.7.
CAS number. 79559-97-0.
7 Medicine Schedule (Poisons Standard)
S4 - Prescription Only Medicine.
Summary Table of Changes
https://stagingapi.mims.com/au/public/v2/images/fulltablegif/SERTAPST.gif