AVELOX® TABLETS
| Consumer Medicine Information (CMI) summary |
The full CMI on the next page has more details. If you are worried about using this medicine, speak to your doctor or pharmacist.
| 1. Why am I using AVELOX tablets? |
AVELOX tablets contains the active ingredient moxifloxacin. AVELOX is used in adults for the treatment of bacterial infections of the lungs, airways and sinuses. For more information, see Section 1. Why am I using AVELOX tablets? in the full CMI.
| 2. What should I know before I use AVELOX tablets? |
Do not use if you have ever had an allergic reaction to AVELOX tablets or any of the ingredients listed at the end of the CMI.
Talk to your doctor if you have any other medical conditions, in particular heart rhythm problems, severe liver problems, seizures, mental illnesses, if you are pregnant or plan to become pregnant or are breastfeeding.
For more information, see Section 2. What should I know before I use AVELOX tablets? in the full CMI.
| 3. What if I am taking other medicines? |
Some medicines may interfere with AVELOX tablets and affect how it works.
The main types of medicines that affect how AVELOX works include warfarin, medicines to treat abnormal heart rhythm, medicines which affect heart rhythm, antacids, multivitamins, mineral supplements and corticosteroids.
A list of these medicines is in Section 3. What if I am taking other medicines? in the full CMI.
| 4. How do I use AVELOX tablets? |
- The usual adult dosage for AVELOX tablets for most infections is one 400 mg tablet once daily for 5 to 10 days.
- Your doctor will determine the duration of time that you take the tablets depending on the type of infection you have.
More instructions can be found in Section 4. How do I use AVELOX tablets? in the full CMI.
| 5. What should I know while using AVELOX tablets? |
| Things you should do |
|
| Things you should not do |
|
| Driving or using machines |
|
| Looking after your medicine |
|
For more information, see Section 5. What should I know while using AVELOX tablets? in the full CMI.
| 6. Are there any side effects? |
Less serious side effects may include redness or pain at the site of injection, headache, dizziness, nausea, vomiting, stomach pains, thrush in the mouth or vagina. Serious side effects may include allergic reactions, palpitations, fainting, seizures, diarrhoea, tendon pain, swelling or rupture, pain, burning, tingling, numbness or weakness that starts or worsens on AVELOX, eyesight problems, changes in mood or thoughts, skin reactions, blistering or peeling, and symptoms of liver damage.
For more information, including what to do if you have any side effects, see Section 6. Are there any side effects? in the full CMI.
AVELOX® TABLETS (AV·e·lox)
Active ingredient(s): moxifloxacin hydrochloride
| Consumer Medicine Information (CMI) |
This leaflet provides important information about using AVELOX tablets. You should also speak to your doctor or pharmacist if you would like further information or if you have any concerns or questions about using AVELOX tablets.
Where to find information in this leaflet:
1. Why am I using AVELOX tablets?
2. What should I know before I use AVELOX tablets?
3. What if I am taking other medicines?
4. How do I use AVELOX tablets?
5. What should I know while using AVELOX tablets?
6. Are there any side effects?
7. Product details
| 1. Why am I using AVELOX tablets? |
AVELOX tablets contains the active ingredient moxifloxacin. AVELOX is an antibiotic belonging to a group of medicines called quinolones. These antibiotics work by killing the bacteria that are causing your infection.
AVELOX tablets are used in adults for the treatment of infections of the lungs, airways and sinuses.
In certain infections, you may require treatment with AVELOX IV injection followed by a course of AVELOX tablets e.g. severe and complicated skin and skin structure infections.
Even if you have read the Consumer Medicine Information for AVELOX IV, you should read this leaflet as well as it contains information specific to the tablets.
AVELOX tablets will not work against infections caused by viruses such as colds or the flu.
| 2. What should I know before I use AVELOX tablets? |
Warnings
Do not use AVELOX tablets if:
- you are allergic to moxifloxacin, or any of the ingredients listed at the end of this leaflet.
Always check the ingredients to make sure you can use this medicine. - you are allergic to other medicines belonging to the quinolone family (e.g. ciprofloxacin, norfloxacin, nalidixic acid).
- you have a condition called ‘QTc prolongation’ which is a type of abnormal heart rhythm
- you are taking medicines to treat arrhythmia – fast, slow or irregular heart beat (e.g. quinidine, procainamide, amiodarone, sotalol)
- you have a blood test that shows lower than normal potassium levels
Check with your doctor if you:
- have any other medical conditions
- take any medicines for any other condition
- you or someone in your family has a history of heart rhythm problems
- are taking any medicine that might affect heart rhythm (e.g. quinidine, procainamide, amiodarone, sotalol, erythromycin, tricyclic antidepressants, antipsychotics)
- have low potassium levels
- have had any condition affecting the brain, particularly if you have ever had a seizure (‘fit’)
- have severe liver problems
- have a condition called myasthenia gravis (a disease causing muscle weakness)
- have or had a mental illness
- have diabetes
During treatment, you may be at risk of developing certain side effects. It is important you understand these risks and how to monitor for them. See additional information under Section 6. Are there any side effects?
Pregnancy and breastfeeding
Check with your doctor if you are pregnant or intend to become pregnant.
AVELOX is not recommended if you are pregnant.
Talk to your doctor if you are breastfeeding or intend to breastfeed.
AVELOX passes into breast milk and may affect your baby.
Children under 18 years old
AVELOX should not be used in children under 18 years of age.
| 3. What if I am taking other medicines? |
Tell your doctor or pharmacist if you are taking any other medicines, including any medicines, vitamins or supplements that you buy without a prescription from your pharmacy, supermarket or health food shop.
Some medicines may interfere with AVELOX tablets and affect how it works.
AVELOX may have an effect on the heart rhythm. If you are using other medicines that affect the heart at the same time as AVELOX there is an increased risk of altering your heart rhythm.
Tell your doctor if you are taking:
- warfarin, a medicine used to stop blood clots. Your doctor should perform INR testing and may adjust your warfarin dose
- medicines used to treat abnormal heart rhythm (e.g. quinidine, procainamide, amiodarone, sotalol)
- medicines that can affect the heart rhythm (erythromycin, tricyclic antidepressants, antipsychotics)
- corticosteroids
These medicines and AVELOX may affect each other or increase the chance of you getting a side effect.
Some medicines may interfere with the absorption of AVELOX tablets. These medicines include:
- antacids, multivitamins, mineral supplements and other medicines containing iron, zinc, magnesium, aluminium or calcium
- sucralfate, a medicine used to treat duodenal or stomach ulcers
- didanosine, a medicine used to treat viral infections
You can still take these medicines while you are taking AVELOX. However, you must take AVELOX at least 2 hours before, or 4 hours after taking any of these medicines to make sure there is no problem with absorption.
Check with your doctor or pharmacist if you are not sure about what medicines, vitamins or supplements you are taking and if these affect AVELOX tablets.
| 4. How do I use AVELOX tablets? |
How much to take
- The usual adult dosage for AVELOX tablets for most infections is one 400 mg tablet once daily for 5 to 10 days.
Some types of infections may require longer treatment. Your doctor will determine the duration of time that you take the tablets depending on the type of infection you have.
It is important that you take the full course of treatment your doctor has prescribed to you - You should not exceed the dose your doctor has prescribed for you. The risk of heart rhythm problems may increase with an increase of the dose.
When to take AVELOX tablets
- AVELOX tablets are usually taken once a day.
- Take your tablet at the same time each day. It can be taken with or without food. It is advisable to drink fluids liberally.
- Swallow the tablet whole with water. Do not chew the tablet.
- Do not take AVELOX at the same time as taking antacids (containing magnesium, calcium or aluminium), multivitamins (containing iron or zinc), sucralfate (a medicine to treat stomach ulcers) or didanosine (a medicine to treat viral infections).
Taking these medicines at the same time as AVELOX can interfere with the absorption of AVELOX tablets and reduce their effectiveness in fighting the infection.
You must take AVELOX at least 2 hours before, or 4 hours after taking any of these medicines.
If you forget to use AVELOX tablets
AVELOX tablets should be used regularly at the same time each day. If you miss your dose at the usual time, and it is:
- 8 hours or more until your next scheduled dose, take your missed dose right away. Then take the next dose at your regular time.
- Less than 8 hours until your next scheduled dose, do not take the missed dose. Take the next dose at your regular time.
If it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to.
Do not take a double dose to make up for the dose you missed.
If you use too much AVELOX tablets
If you think that you have used too much AVELOX, you may need urgent medical attention.
You should immediately:
- phone the Poisons Information Centre
(by calling 13 11 26), or - contact your doctor, or
- go to the Emergency Department at your nearest hospital.
You should do this even if there are no signs of discomfort or poisoning.
| 5. What should I know while using AVELOX tablets? |
Things you should do
- Tell all doctors, dentists and pharmacists who are treating you that you are taking AVELOX.
- If you are about to be started on any new medicine, remind your doctor and pharmacist that you are taking AVELOX tablets.
- Avoid prolonged exposure to ultraviolet radiation and sunlight. AVELOX can make your skin more susceptible to sunburn. Protect your skin when you are in the sun, especially between 10am and 3pm. If you are outdoors, wear protective clothing and use a SPF 30+ sunscreen.
Call your doctor straight away if you:
- become pregnant while you are taking AVELOX
- develop an allergic reaction (e.g. skin rash) while taking AVELOX, even following a single dose. Stop taking it and tell your doctor.
- develop diarrhoea. Tell your doctor even if it occurs several weeks after you have stopped taking AVELOX.
- feel any discomfort, pain, swelling or inflammation of a tendon.
- experience palpitations (fast or irregular heart beats) or fainting spells during treatment
- experience symptoms of depression or self-harming behaviour. AVELOX should be discontinued.
- develop pain, burning, tingling, numbness or weakness in any part of the body. AVELOX should be discontinued immediately.
- develop photosensitivity (getting sunburnt very easily)
Remind any doctor, dentist or pharmacist you visit that you are using AVELOX tablets.
Things you should not do
- Do not give your AVELOX tablets to anyone else, even if they have the same condition as you.
- Do not use AVELOX to treat other conditions unless your doctor tells you to.
- Do not change the dose.
- Do not stop taking your tablets because you are feeling better, unless your doctor told you to do so. If you do not complete the full course prescribed by your doctor, some of the bacteria causing your infection may not be killed. These bacteria may continue to grow and multiply so that your infection may not clear up completely or it may return.
Driving or using machines
Be careful before you drive or use any machines or tools until you know how AVELOX tablets affects you.
AVELOX tablets may cause dizziness or faintness in some people. Be careful before you drive or use any machines or tools until you know how AVELOX tablets affect you.
Drinking alcohol
Tell your doctor if you drink alcohol.
Looking after your medicine
Keep your tablets in the blister pack until it is time to take them. If you take the tablets out of the box or the blister pack, they may not keep well.
Store it in a cool dry place away from moisture, heat or sunlight where the temperature says below 25°C.
Do NOT store it in the bathroom or near a sink, or in the car or on window sills.
Keep it where young children cannot reach it.
Getting rid of any unwanted medicine
If you no longer need to use this medicine or it is out of date, take it to any pharmacy for safe disposal.
If the packaging is damaged, return it to your pharmacist for disposal.
| 6. Are there any side effects? |
All medicines can have side effects. If you do experience any side effects, most of them are minor and temporary. However, some side effects may need medical attention.
See the information below and, if you need to, ask your doctor or pharmacist if you have any further questions about side effects.
Less serious side effects
| Less serious side effects | What to do |
Nervous system-related:
| Speak to your doctor if you have any of these less serious side effects and they worry you. |
Serious side effects
| Serious side effects | What to do |
Immune-related:
suddenly feeling unwell or sick and develop symptoms such as:
| Call your doctor straight away, or go straight to the Emergency Department at your nearest hospital if you notice any of these serious side effects. |
Tell your doctor or pharmacist if you notice anything else that may be making you feel unwell.
Other side effects not listed here may occur in some people.
Reporting side effects
After you have received medical advice for any side effects you experience, you can report side effects to the Therapeutic Goods Administration online at www.tga.gov.au/reporting-problems. By reporting side effects, you can help provide more information on the safety of this medicine.
Always make sure you speak to your doctor or pharmacist before you decide to stop taking any of your medicines.
| 7. Product details |
This medicine is only available with a doctor's prescription.
What AVELOX tablets contains
| Active ingredient (main ingredient) | moxifloxacin (as hydrochloride) 400 mg |
| Other ingredients (inactive ingredients) |
|
| Potential allergens | lactose monohydrate |
Do not take this medicine if you are allergic to any of these ingredients.
What AVELOX tablets looks like
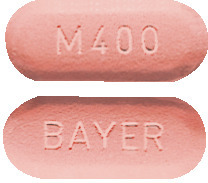
AVELOX tablets are dull red, oblong, film-coated, convex tablets marked with M 400 on one side and BAYER on the other side.
It is available in blister packs of 5 tablets (Aust R 75766).
Who distributes AVELOX tablets
Bayer Australia Ltd
ABN 22 000 138 714
875 Pacific Highway
Pymble NSW 2073
See TGA website (www.ebs.tga.gov.au) for latest Australian Consumer Medicine Information.
® Registered Trademark of Bayer Group, Germany
© Bayer Australia Ltd
All rights reserved.
This leaflet was prepared in July 2023.
Published by MIMS October 2023