AZEP® NASAL SPRAY
Active ingredient(s): azelastine hydrochloride
| Consumer Medicine Information (CMI) |
This leaflet provides important information about using AZEP NASAL SPRAY. You should also speak to your doctor or pharmacist if you would like further information or if you have any concerns or questions about using AZEP NASAL SPRAY.
Where to find information in this leaflet:
1. Why am I using AZEP NASAL SPRAY?
2. What should I know before I use AZEP NASAL SPRAY?
3. What if I am taking other medicines?
4. How do I use Azep Nasal Spray?
5. What should I know while using AZEP NASAL SPRAY?
6. Are there any side effects?
7. Product details
| 1. Why am I using AZEP NASAL SPRAY? |
AZEP NASAL SPRAY is used to relieve runny nose, sneezing, itching or blocked nose caused by allergies, such as to pollen (hay fever) house dust mites or pet hair.
AZEP NASAL SPRAY belongs to a group of medicines called antihistamines. It works by blocking the action of histamine and other substances produced by the body, which are causing your allergies.
AZEP NASAL SPRAY is not addictive.
| 2. What should I know before I use AZEP NASAL SPRAY? |
Warnings
Do not use AZEP NASAL SPRAY if you have an allergy to AZEP NASAL SPRAY or any of the ingredients listed at the end of this leaflet
Always check the ingredients to make sure you can use this medicine.
Do not give AZEP NASAL SPRAY to a child under 5 years, unless directed by the child's doctor.
The safety and effectiveness of AZEP NASAL SPRAY in children under 5 years have not been established.
Check with your doctor or pharmacist if you:
- have any other medical conditions, especially kidney disease
- take any medicines for any other condition
During treatment, you may be at risk of developing certain side effects. It is important you understand these risks and how to monitor for them. See additional information under Section 6. Are there any side effects?
Pregnancy and breastfeeding
Check with your doctor or pharmacist if you are pregnant or intend to become pregnant.
Talk to your doctor or pharmacist if you are breastfeeding or intend to breastfeed.
| 3. What if I am taking other medicines? |
Tell your doctor or pharmacist if you are taking any other medicines, including any medicines, vitamins or supplements that you buy without a prescription from your pharmacy, supermarket or health food shop.
Some medicines and AZEP NASAL SPRAY may interfere with each other if a significant amount of the nasal spray is absorbed in your body. These include:
- cimetidine, a medicine used to treat stomach ulcers and some other stomach conditions.
Check with your doctor or pharmacist if you are not sure about what medicines, vitamins or supplements you are taking and if these affect AZEP NASAL SPRAY.
| 4. How do I use AZEP NASAL SPRAY? |
How much to use
- Use one spray of AZEP NASAL SPRAY in each nostril, every 12 hours as necessary.
- Follow the instructions provided with the medicine.
- Do not exceed the recommended dosage.
- AZEP NASAL SPRAY can be used for up to 6 months for seasonal and non-seasonal allergies.
When to use AZEP NASAL SPRAY
- AZEP NASAL SPRAY should be used only when necessary. Do not use a double dose to make up for the dose that you missed.
How to use AZEP NASAL SPRAY
Blow your nose gently, to clear away any mucus.
- Remove the protective cap.
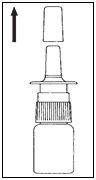
- Before first use, prime the spray by pressing the pump 2-3 times until an even spray emerges.
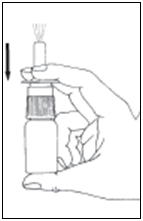
- Tilt your head forwards (look at your toes). Spray once into each nostril, closing the other nostril with one finger. Sniff slowly and gently.
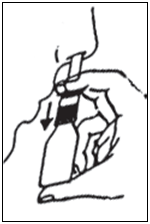
- Wipe the nozzle and replace the protective cap.
Prime the pump again if you have not used the spray for 3 or more days.
If you use too much AZEP NASAL SPRAY
If you think that you have used too much AZEP NASAL SPRAY, you may need urgent medical attention.
You should immediately:
- phone the Poisons Information Centre
(Australia telephone 13 11 26) for advice, or - contact your doctor, or
- go to the Emergency Department at your nearest hospital.
You should do this even if there are no signs of discomfort or poisoning.
| 5. What should I know while using AZEP NASAL SPRAY? |
Things you should do
Remind any doctor, dentist or pharmacist you visit that you are using AZEP NASAL SPRAY.
If you become pregnant while using AZEP NASAL SPRAY tell your doctor or pharmacist.
Things you should not do
- Do not use AZEP NASAL SPRAY to treat any other complaints unless your doctor or pharmacist tells you to.
- Do not give AZEP NASAL SPRAY to anyone else, even if they have the same condition as you.
Driving or using machines
Be careful before you drive or use any machines or tools until you know how AZEP NASAL SPRAY affects you.
AZEP NASAL SPRAY is unlikely to make you feel drowsy. However, if you are drowsy, do not drive a car or operate machinery.
Drinking alcohol
Tell your doctor or pharmacist if you drink alcohol.
If you drink alcohol, any drowsiness may be worse.
Looking after your medicine
- Keep your AZEP NASAL SPRAY 5 mL in a cool dry place where the temperature stays below 25°C.
- Keep your AZEP NASAL SPRAY 20 mL in a cool dry place where the temperature stays below 30°C.
- Do not put AZEP NASAL SPRAY in the fridge.
Follow the instructions in the carton on how to take care of your medicine properly.
Store it in a cool dry place away from moisture, heat or sunlight; for example, do not store it:
- in the bathroom or near a sink, or
- in the car or on window sills.
Keep it where young children cannot reach it.
When to discard your medicine (as relevant)
Do not use AZEP NASAL SPRAY for longer than 6 months after the bottle was first opened.
Getting rid of any unwanted medicine
If you no longer need to use this medicine or it is out of date, take it to any pharmacy for safe disposal.
Do not use this medicine after the expiry date.
| 6. Are there any side effects? |
All medicines can have side effects. If you do experience any side effects, most of them are minor and temporary. However, some side effects may need medical attention.
See the information below and, if you need to, ask your doctor or pharmacist if you have any further questions.
Less serious/common side effects
| Less serious side effects | What to do |
| Speak to your doctor or pharmacist if you have any of these less serious side effects and they worry you. Drinking a flavoured drink a few minutes after using AZEP NASAL SPRAY may help remove any bitter taste. |
Tell your doctor or pharmacist if you notice anything else that may be making you feel unwell.
Other side effects not listed here may occur in some people.
Reporting side effects
After you have received medical advice for any side effects you experience, you can report side effects to the Therapeutic Goods Administration online at www.tga.gov.au/reporting-problems. By reporting side effects, you can help provide more information on the safety of this medicine.
| 7. Product details |
This medicine is available over-the-counter without a doctor's prescription.
What AZEP NASAL SPRAY contains
| Active ingredient (main ingredient) | Azelastine hydrochloride 0.1% w/v [One spray contains 125 microgram of azelastine (as hydrochloride)] |
| Other ingredients (inactive ingredients) |
|
| Potential allergens | N/A |
Do not take this medicine if you are allergic to any of these ingredients.
What AZEP NASAL SPRAY looks like
AZEP NASAL SPRAY (AUST R 104853) is a clear, nearly colourless liquid in a brown glass bottle fitted with a spray pump and a cap.
The 5 mL bottle contains 35 metered sprays.
The 20 mL bottle contains 140 metered sprays.
Who distributes AZEP NASAL SPRAY
Viatris Pty Ltd
Level 1, 30 The Bond
30-34 Hickson Road
Millers Point, NSW 2000
www.viatris.com.au
Phone: 1800 274 276
This leaflet was prepared in October 2021.
AZEP® NASAL SPRAY is a Viatris company trade mark
AZEP NASAL SPRAY_cmi\Oct21/00
Published by MIMS January 2022
