1 Name of Medicine
Potassium chloride and sodium chloride.
6.7 Physicochemical Properties
Potassium chloride.
Molecular formula: KCl.
Molecular Weight: 74.55.
Appearance: colourless or white crystal.
Solubility: freely soluble in water.
Sodium chloride.
Molecular formula: NaCl.
Molecular Weight: 58.44.
Appearance: colourless or white crystal.
Solubility: freely soluble in water and practically insoluble in anhydrous ethanol.
CAS number.
Potassium chloride.
CAS No.: 7447-40-7.
Sodium chloride.
CAS No.: 7647-14-5.2 Qualitative and Quantitative Composition
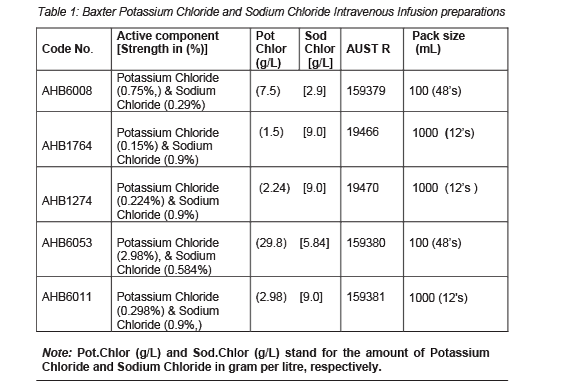
Baxter Potassium Chloride and Sodium Chloride Intravenous (IV) Infusion is a sterile, non-pyrogenic solution. The amounts of potassium chloride and sodium chloride dissolved in water for injections are shown in Table 1. Hydrochloric acid may be added for pH adjustment. They do not contain an antimicrobial agent or added buffer and have a pH of 4.0-7.0. The products are isotonic solutions except for potassium chloride 30 mmol and 0.9% sodium chloride and potassium chloride 40 mmol and 0.9% sodium chloride, these are hypertonic solutions.
 For the full list of excipients, see Section 6.1 List of Excipients.
For the full list of excipients, see Section 6.1 List of Excipients.
3 Pharmaceutical Form
Clear colourless solution for intravenous infusion.
5 Pharmacological Properties
5.1 Pharmacodynamic Properties
Mechanism of action.
Baxter Potassium Chloride and Sodium Chloride IV Infusion is mainly intended for the treatment of potassium depletion. Thus, the mode of action of these formulations should be looked at from that viewpoint. Potassium is a major cation of the intracellular fluid (160 mmol/litre of intracellular water) found primarily in muscle cells. It functions principally in the maintenance of acid-base balance; isotonicity and electrodynamic characteristics of the cells. In contrast, sodium is the major cation of the extracellular fluid (135 to 145 mmol/litre) and functions principally in the control of water distribution, fluid and electrolyte balance and osmotic pressure of body fluids.
Na-K-ATPase membrane bound enzymes regulate the passage of potassium against a higher potassium concentration in the cells. Potassium participates in carbohydrate utilisation, protein synthesis, and is critical in the regulation of nerve conduction and muscle contraction, particularly in the cardiac muscle.
Chloride, the major extracellular anion, closely follows the physiological disposition of sodium cation in maintenance of acid-base balance, isotonicity and electrodynamic characteristics of the cells. An increase of chloride concentration may result in a decrease of bicarbonate level, which leads to plasma acidosis, as shown by the charge-neutrality of the cells by the following equation. That is, Na+ = Cl- + HCO3- + [anion gap]-, where pH is related to equation, pH = pKH2CO3 + log [HCO3]/[H2CO3]. The anion gap is called "unmeasured anion", thus, Baxter Potassium Chloride and Sodium Chloride IV Infusion preparation has a value as a source of water, and electrolytes where kidney may excrete potassium up to (80-90) mmol daily (see Section 2 Qualitative and Quantitative Composition, Table 1).
Daily requirements of potassium are between 800 mg to 1.2 g.
Clinical trials.
No data available.
5.2 Pharmacokinetic Properties
As Baxter Potassium Chloride and Sodium Chloride IV Infusion is directly administered to the systemic circulation, the bioavailability (absorption) of the active components is complete (100 per cent). From vascular system potassium ions first enter the extracellular/interstitial fluid, which then are pumped into the cells against concentration gradient by the Na-K-ATPase active transport mechanism.
The level of potassium in the body is regulated by glomerular filtration and distal tubular secretion. Potassium excretion site is accompanied by sodium and water reabsorption back into systemic circulation. Thus, kidney constantly adjusts the sodium and potassium level through this mechanism. The loss of sodium can be reduced to zero by increasing potassium and hydrogen ion excretion. Hormones, ADH (antidiuretic hormone) and aldosterone control the kidney function in reabsorption of water and excretion of potassium, respectively.
The capacity of the kidney to conserve potassium is poor and some urinary excretion of potassium continues even when there is severe depletion. Some potassium is excreted in the faeces and small amounts may be excreted in sweat.
5.3 Preclinical Safety Data
Genotoxicity.
The active ingredients, potassium chloride and sodium chloride are not mutagenic.
Carcinogenicity.
The active ingredients, potassium chloride and sodium chloride are not carcinogenic.4.1 Therapeutic Indications
The Baxter Potassium Chloride and Sodium Chloride IV Infusion is indicated as a source of water and to restore electrolyte balance as required by the patient's clinical condition, such as hypokalaemia.
4.3 Contraindications
Baxter Potassium Chloride and Sodium Chloride IV Infusion is contraindicated in patients with:
known hypersensitivity to the product;
documented hyperkalaemia, hyperchloraemia or hypernatraemia;
potassium retention;
congestive heart failure;
severe impairment of renal function;
acidosis;
haemolysis;
Addison's disease;
in conjunction with potassium sparing diuretics;
clinical states in which the administration of sodium and chloride is detrimental.
4.4 Special Warnings and Precautions for Use
Special warnings.
High dose or high speed infusion must be performed under continuous ECG monitoring. To avoid potassium intoxication, Baxter Potassium Chloride and Sodium Chloride IV Infusion must not be infused rapidly. Administration should be carried out under regular and careful surveillance. Regular monitoring of clinical status, plasma electrolyte concentrations, plasma creatinine levels, BUN level, acid-base balance and ECG is essential in patients receiving potassium therapy, particularly those with cardiac or renal impairment. Adequate urine flow should be ensured and fluid balance should be monitored.
When infusing Baxter Potassium Chloride and Sodium Chloride IV Infusion; care must be taken to prevent paravenous administration or extravasation because such solutions may be associated with tissue damage, which may be severe and include vascular, nerve and tendon damage, leading to surgical intervention, including amputation. Secondary complications including pulmonary embolism from thrombophlebitis have been reported as a consequence of tissue damage from potassium chloride.
Sodium salts should be administered with caution to patients with hypertension, heart failure, peripheral or pulmonary oedema, impaired renal function, pre-eclampsia, or other conditions associated with sodium retention (also see Section 4.5 Interactions with Other Medicines and Other Forms of Interactions).
Rapid correction of hypernatraemia and hyponatraemia is potentially dangerous (risk of serious neurologic complications).
In order to reduce risks of thrombophlebitis, it is recommended to change the injection site every 24 hrs.
In a dilute condition, osmolarity is approximately the same with osmolality.
The addition of potassium chloride into an isotonic sodium chloride solution renders Baxter Potassium Chloride and Sodium Chloride IV Infusion to be hypertonic (see Section 2 Qualitative and Quantitative Composition, Table 1, for osmolarity of solutions). Administration of substantially hypertonic solution may lead to a wide variety of complications, such as crenation (shrinkage) of red blood cells and general cellular dehydration.
Hypersensitivity reactions.
Hypersensitivity/infusion reactions including anaphylaxis have been reported with other products containing potassium chloride and sodium chloride. Stop the infusion immediately if signs or symptoms of hypersensitivity/infusion reactions develop. Appropriate therapeutic countermeasures must be instituted as clinically indicated.
Hyperkalaemia.
Potassium salts should be administered with considerable care to patients with cardiac disease or conditions predisposing to hyperkalaemia and/or associated with increased sensitivity to potassium such as patients with:
renal impairment or adrenocorticol insufficiency;
acute dehydration;
extensive tissue injury or burns;
certain cardiac disorders such as congestive heart failure or AV block (especially if they receive digitalis). In patients under digitalis therapy, regular monitoring of the plasma potassium level is mandatory;
potassium-aggravated skeletal muscle channelopathies (e.g. hyperkalaemic periodic paralysis, paramyotonia congenita, and potassium-aggravated myotonia/paramyotonia).
Baxter Potassium Chloride and Sodium Chloride IV Infusion should be administered with caution to patients who are at risk of experiencing hyperosmolality, acidosis, or undergo correction of alkalosis (conditions associated with a shift of potassium from intracellular to extracellular space) and patients treated concurrently or recently with agents or products that can cause hyperkalaemia (see Section 4.5 Interactions with Other Medicines and Other Forms of Interactions). Close monitoring, careful dose selection and adjustment is required particularly in high risk patients.
Hyperkalaemia can cause cardiac conduction disorders (including complete heart block) and other cardiac arrhythmias at any time during infusion. Continuous ECG monitoring is performed to aid in the detection of cardiac arrhythmias due to a sudden increase in serum potassium concentration (e.g. when potassium infusion is started) or transient or sustained hyperkalaemia (see Section 4.8 Adverse Effects (Undesirable Effects); Section 4.9 Overdose).
Frequently, mild or moderate hyperkalaemia is asymptomatic and may be manifested only by increased serum potassium concentrations and possibly characteristic ECG changes. However, fatal arrhythmias can develop at any time during hyperkalaemia. Serum potassium levels are not necessarily indicative of tissue potassium levels.
Use in patients at risk of sodium retention, fluid overload and oedema.
Baxter Potassium Chloride and Sodium Chloride IV Infusion should be used with particular caution, in patients with or at risk for: hypernatraemia; hyperchloremia; metabolic acidosis; hypervolaemia; conditions that may cause sodium retention, fluid overload and oedema (central and peripheral).
Risk of serum electrolytes and water imbalance.
Depending on the volume and rate of infusion and depending on a patient's underlying clinical condition, the intravenous administration of Baxter Potassium Chloride and Sodium Chloride IV Infusion can cause:
fluid and/or solute overloading resulting in dilution of the serum electrolyte concentrations;
electrolyte disturbances such as: hypernatraemia, hyponatraemia;
acid-base imbalance;
overhydration/ hypervolaemia, congested states, including central (e.g. pulmonary congestion) and peripheral oedema.
The risk of dilution states is inversely proportional to the electrolyte concentrations of the injections. The risk of solute overload causing congested states with peripheral and pulmonary oedema is directly proportional to the electrolyte concentrations of the injections.
Regarding medications that increase the risk of hyponatraemia or sodium and fluid retention, see Section 4.5 Interactions with Other Medicines and Other Forms of Interactions.
In patients with diminished renal function, administration of Baxter Potassium Chloride and Sodium Chloride IV Infusion may result in sodium or potassium retention. Clinical evaluation and periodic laboratory determinations may be necessary to monitor changes in fluid balance, electrolyte concentration and acid-base balance during prolonged parenteral therapy or whenever the condition of the patient or the rate of administration warrants such evaluation.
Hyponatraemia.
Monitoring of serum sodium is particularly important for hypotonic fluids (see Section 2 Qualitative and Quantitative Composition, Table 1, for osmolarity of the solutions). High volume infusion must be used under specific monitoring in patients with cardiac or pulmonary failure, and in patients with non-osmotic vasopressin release (including SIADH), due to the risk of hospital-acquired hyponatraemia.
Baxter Potassium Chloride and Sodium Chloride IV Infusion should be used with particular caution in patients with or at risk of hyponatraemia, for example:
in children;
in elderly patients;
in women; postoperatively;
in persons with psychogenic polydipsia; in patients treated with medications that increase the risk of hyponatraemia (such as certain antiepileptic and psychotropic medications).
The risk for developing hyponatraemic encelopathy is increased, for example:
in paediatric patients (≤ 16 years of age);
in women (in particular, premenopausal women);
in patients with hypoxemia;
in patients with underlying central nervous system disease.
Hyponatraemia can lead to acute hyponatraemic encephalopathy (brain oedema) characterised by headache, nausea, seizures, lethargyand vomiting which may lead to command death. Patients with brain oedema are at particular risk of severe, irreversible and life-threatening brain injury. Acute symptomatic hyponatraemic encephalopathy is considered a medical emergency.
Use in patients at risk of severe renal impairment.
Baxter Potassium Chloride and Sodium Chloride IV Infusion should be administered with particular caution to patients at risk of severe renal impairment. In such patients, administration of Baxter Potassium Chloride and Sodium Chloride IV Infusion may result in sodium retention, fluid overload, and/or may predispose to hyperkalaemia.
Risk of air embolism.
Do not connect flexible plastic containers in series in order to avoid air embolism due to possible residual air contained in the primary container.
Pressurising intravenous solutions contained in flexible containers to increase flow rates can result in air embolism if the residual air in the container is not fully evacuated prior to administration.
Use of a vented intravenous administration set with the vent in the open position could result in air embolism. Vented intravenous administration sets with the vent in the open position should not be used with flexible plastic containers.
Use in the elderly.
When selecting the type of infusion solution and the volume/rate of infusion for a geriatric patient, consider that geriatric patients are generally more likely to have cardiac, renal, hepatic, and other diseases and/or concomitant drug therapy.
Paediatric use.
These solutions have not been developed for use in children, and age specific paediatric protocols must be consulted.
The infusion rate and volume depends on the age, weight, clinical and metabolic conditions of the patient, concomitant therapy, and should be determined by a physician experienced in paediatric intravenous fluid therapy. Paediatric use requires the application of specific institutional protocols to calculate appropriate dose rates for individual patients.
Children (including neonates and older children) are at increased risk of developing hyponatraemia as well as for developing hyponatraemic encephalopathy. The infusion of Baxter Potassium Chloride and Sodium Chloride IV Infusion together with the non-osmotic secretion of ADH may result in hyponatraemia.
Plasma electrolyte concentrations should be closely monitored in the paediatric population.
Effects on laboratory tests.
The effect of this medicine on laboratory tests has not been established.4.5 Interactions with Other Medicines and Other Forms of Interactions
Caution is advised in patients treated with lithium. Renal sodium and lithium clearance may be increased during administration of Baxter Potassium Chloride and Sodium Chloride IV Infusion and this can result in decreased lithium levels.
Solutions containing potassium should be used with caution in patients treated concurrently or recently with agents or products that can cause hyperkalaemia or increase the risk of hyperkalaemia (e.g. potassium sparing diuretics including amiloride, spironolactone and triamterene, ACE inhibitors, angiotensin II receptor antagonists, cyclosporin, tacrolimus and drugs that contain potassium such as potassium salts of penicillin). Administration of potassium in patients treated with such agents is associated with an increased risk of severe and potentially fatal hyperkalaemia particularly in the presence of other risk factors for hyperkalaemia.
Caution is advised when administering Baxter Potassium Chloride and Sodium Chloride IV Infusion to patients treated with drugs leading to an increased vasopressin effect. The below listed drugs increase the vasopressin effect, leading to reduced renal electrolyte free water excretion and may increase the risk of hyponatraemia following treatment with IV fluids. (See Section 4.4 Special Warnings and Precautions for Use; Section 4.8 Adverse Effects (Undesirable Effects)):
Drugs stimulating vasopressin release such as chlorpropamide, clofibrate, carbamazepine, vincristine, selective serotonin reuptake inhibitors (SSRIs), 3.4-methylenedioxy-N-methamphetamine, ifosfamide, antipsycotics, opioids.
Drugs potentiating vasopressin action such as chlorpropamide, non-steroidal antiinflammatories (NSAIDS), cyclophosphamide.
Vasopressin analogues such as desmopressin, oxytocin, vasopressin, terlipressin.
Baxter Potassium Chloride and Sodium Chloride IV Infusion should be used with particular caution in patients on concomitant medications that may increase the risk of sodium and fluid retention, such as corticosteroids. Corticosteroids and corticotropin are associated with the retention of sodium and water, with oedema and hypertension.
Potassium chloride is not compatible with mannitol 20%, sodium bicarbonate and colloidal solutions.
Baxter Potassium Chloride and Sodium Chloride IV Infusion should be administered with caution in patients on concomitant medications that increase the risk of hyponatraemia such as diuretics, and certain antiepileptic and psychotropic medications.
The safety of the Viaflex plastic container used to contain the Baxter Potassium Chloride and Sodium Chloride IV Infusion has been confirmed in tests in animals according to the USP biological tests for plastic container, as well as by tissue culture toxicity studies. Nevertheless, care should be exercised regarding a possible incompatibility outcome resulting either from the interaction between the plastic container or active ingredients and the added therapeutic substances (see Section 4.2 Dose and Method of Administration).
The introduction of additives to any solution, regardless of type of container, requires special attention to ensure that no incompatibilities result. While some incompatibilities are readily observed, one must be aware that subtle physical, chemical and pharmacological incompatibilities can occur. The medical literature, the package insert and other available sources of information should be reviewed for thorough understanding of possible incompatibility problems. Additives known or determined to be incompatible should not be used.
See Section 6.2 Incompatibilities.
4.6 Fertility, Pregnancy and Lactation
Effects on fertility.
No data available.
(Category C)
Animal reproduction studies have not been conducted with Baxter Potassium Chloride and Sodium Chloride IV Infusion. It is also not known whether these dosage forms can cause foetal harm when administered to a pregnant woman or can affect reproduction capacity. There are no adequate data from the use of Baxter Potassium Chloride and Sodium Chloride IV Infusion in pregnant women. Physicians should carefully consider the potential risks and benefits for each specific patient before administering Baxter Potassium Chloride and Sodium Chloride IV Infusion.
Safety in lactation has not been established. There are no adequate data from the use of Baxter Potassium Chloride and Sodium Chloride IV Infusion in lactating women. Physicians should carefully consider the potential risks and benefits for each specific patient before administering Baxter Potassium Chloride and Sodium Chloride IV Infusion.4.8 Adverse Effects (Undesirable Effects)
Adverse reactions to potassium containing solutions include hyperkalaemia, paraesthesia of the extremities, flaccid paralysis, mental confusion, hypotension, cardiac arrhythmias, heart block, ECG abnormalities and cardiac arrest.
Adverse reactions which may occur because of the solution or the technique of administration, include fever response, infection at the site of injection, venous thrombosis or phlebitis extending from the site of injection, extravasation and hypervolemia. If an adverse reaction does occur, discontinue the infusion, evaluate the patient, institute appropriate therapeutic countermeasures and save the remainder of the fluid for examination if deemed necessary.
Post-marketing adverse reactions.
The following adverse reactions have been reported in the post-marketing experience listed by MedDRA System Organ Class (SOC).
Immune system disorders.
Hypersensitivity, as manifested by rash and angioedema.
Metabolism and nutrition disorder.
Hyperkalaemia, hyponatraemia, hypernatraemia, acidosis hyperchloremic, fluid overload.
Cardiac disorders.
Cardiac arrest*, asystole*, ventricular fibrillation*, bradycardia (*as manifestation of rapid intravenous administration and/or of hyperkalaemia).
Respiratory, thoracic and mediastinal disorders.
Dyspnoea.
General disorders and administration site conditions.
Chest pain, chills, infusion site pain, infusion site irritation, burning sensation.
Other adverse reaction associated with administration of Baxter Potassium Chloride and Sodium Chloride IV Infusion include:
in association with extravasation: skin necrosis, skin ulcer, soft tissue necrosis, muscle necrosis, nerve injury, tendon injury and vascular injury;
infusion site thrombosis, infusion site phlebitis, infusion site swelling and infusion site erythema.
Other reactions (class reactions).
Other adverse reactions reported with other similar products include:
Nervous system disorders.
Hyponatraemic encephalopathy.
Reporting suspected adverse effects.
Reporting suspected adverse reactions after registration of the medicinal product is important. It allows continued monitoring of the benefit-risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions at www.tga.gov.au/reporting-problems.4.2 Dose and Method of Administration
To be used as directed by the physician for intravenous use only. The choice of the specific Baxter Potassium Chloride and Sodium Chloride IV Infusion formulation, dosage, volume, rate and duration of administration is dependent upon the age, weight, clinical and biological (acid-base balance) condition of the patient, concomitant therapy and laboratory determinations. Additional electrolyte supplementation may be indicated according to the clinical needs of the patient. Administration should be determined by a physician experienced in intravenous fluid therapy. A rate-limiting device such as a rate- controlled infusion pump should be used to prevent unintentional bolus doses of solutions containing potassium chloride. Institutional guidelines for administration of intravenous potassium should be followed.
Intravenous potassium should be administered in a large peripheral or central vein to diminish the risk of causing sclerosis. If infused through a central vein, be sure the catheter is not in the atrium or ventricle to avoid localised hyperkalaemia. The Baxter Potassium Chloride and Sodium Chloride IV Infusion range have a pH of 4.0 - 7.0 and their osmolarity are shown in Table 1 (see Section 2 Qualitative and Quantitative Composition). The osmolarity of a final admixed infusion solution must be taken into account when peripheral administration is considered. Hyperosmolar solutions may cause venous irritation and phlebitis. Thus, clinically significant hyperosmolar solutions are recommended to be administered through a large central vein, for rapid dilution of the hyperosmolar solution.
Solutions containing potassium should be administered under the following conditions:
The 100 mL presentation must be infused over at least 1 hour.
The maximum time over which infusion may occur is 12 hours for the 100 mL product, and 24 hours for the 1000 mL presentations.
The recommended administration rate should not exceed 20 mmol/hour and not exceed 80 mmol for a 24-hour period (= 6 g KCl/24 hours).
Paediatric use requires the application of specific institutional protocols to calculate appropriate dose rates for individual patients. Do not exceed 3 mmol/kg/day.
Do not connect flexible plastic containers in series in order to avoid air embolism due to possible residual air contained in the primary container.
Baxter Potassium Chloride and Sodium Chloride IV Infusion is intended for intravenous administration using sterile equipment and strict aseptic technique. Parenteral drug products should be inspected visually for particulate matter and discolouration prior to administration wherever solution and container permit. Do not administer unless solution is clear and seal is intact.
The solutions contain no antimicrobial agents, and are for single use in only one patient. Unused portions must be discarded.
The volume in the 1000 mL bags, but not the 100 mL bags, will accommodate additives. Do not add supplementary medication.
Check for minute leaks by squeezing inner bag firmly. If leaks are found, discard solution, as sterility may be impaired.
Additives may be incompatible. When introducing additives to Baxter Potassium Chloride and Sodium Chloride IV Infusion, the instructions for use of the medication to be added and other relevant literature must be consulted. Before adding a substance or medication, verify that it is soluble and stable in Baxter Potassium Chloride and Sodium Chloride IV Infusion, and that the pH range of the solution is appropriate. Only those additives known to be compatible can be added to these infusions. Consult with pharmacist, if available. If in the informed judgment of the physician, it is deemed advisable to introduce additives, use aseptic technique. Add additives to inverted container (ports uppermost) with a 0.63 to 0.80 mm needle. Squeeze ports and mix thoroughly. After addition, if there is a discoloration and/or the appearance of precipitates, insoluble complexes or crystals, do not use.
In case of damage, the container should be discarded. Do not store solutions containing additives.
Discard any unused portion. For single use only.
Monitoring.
Adequate urine flow must be ensured and careful monitoring of electrolyte concentrations and ECG is essential (see Section 4.4 Special Warnings and Precautions for Use).4.7 Effects on Ability to Drive and Use Machines
There is no information on the effects of Baxter Potassium Chloride and Sodium Chloride IV Infusion on the ability to operate an automobile or other heavy machinery.
4.9 Overdose
Excess administration of Baxter Potassium Chloride and Sodium Chloride IV Infusion can cause:
Hyponatraemia (which can lead to CNS manifestations including seizures, coma, cerebral edema and death).
Hypernatraemia, especially in patients with severe renal impairment.
Hyperkalaemia. Potassium overdose can cause potentially fatal hyperkalaemia. The clinical signs and symptoms of hyperkalaemia include:
disturbances in cardiac conduction and arrhythmias, including bradycardia, heart block, asystole, ventricular tachycardia, ventricular fibrillation;
hypotension, cold skin, grey pallor and peripheral collapse with fall in blood pressure;
muscle weakness up to and including muscular and respiratory paralysis, paraesthesia of extremities;
gastrointestinal symptoms (ileus, nausea, vomiting, abdominal pain);
mental confusion.
Fluid overload (which can lead to central and/or peripheral oedema). (See Section 4.4 Special Warnings and Precautions for Use; Section 4.8 Adverse Effects (Undesirable Effects)).
Extremely high serum potassium concentrations (8-11 mmol/L) may cause death from cardiac depression, arrhythmias or arrest.
Frequently, mild or moderate hyperkalaemia is asymptomatic and may be manifested only by increased serum potassium concentrations and, possibly, characteristic electrocardiographic changes. However, fatal arrhythmias can develop at any time.
In addition to arrhythmias and conduction disorders, the ECG shows progressive changes that occur with increasing potassium levels. Possible changes include: peaking of T waves; loss of P waves; QRS widening.
The presence of any ECG findings that are suspected to be caused by hyperkalaemia should be considered a medical emergency.
When assessing an overdose, any additives in the solution must also be considered. The effects of an overdose may require immediate medical attention and treatment. Interventions include discontinuation of Baxter Potassium Chloride and Sodium Chloride IV Infusion administration, dose reduction, and other measures as indicated for the specific clinical constellation.
If hyperkalaemia is present or suspected, discontinue the infusion immediately and institute close ECG, laboratory and other monitoring and, as necessary, corrective therapy to reduce serum potassium levels.
Lowering of the potassium level should be approached with thorough consideration on adverse effects that may occur, in particular with digitalised patients.
A state of hypokalaemia increases the risk of digitalis toxicity. Plasma electrolyte abnormalities (hypomagnesemia, hypokalaemia and metabolic alkalosis) also contribute to the clinical toxicity even at normal digoxin plasma level. Thus, caution should be exercised when lowering the potassium level in a digitalised patient.
For information on the management of overdose, contact the Poisons Information Centre on 13 11 26 (Australia).
7 Medicine Schedule (Poisons Standard)
Unscheduled.
6 Pharmaceutical Particulars
6.1 List of Excipients
Water for injections; hydrochloric acid (for pH adjustment).
6.2 Incompatibilities
Additives may be incompatible. Consult with a pharmacist, if available. Check additive compatibility with both the solution and container prior to use. Those additives known or determined to be incompatible should not be used (see Section 4.2 Dose and Method of Administration; Section 4.5 Interactions with Other Medicines and Other Forms of Interactions).
6.3 Shelf Life
In Australia, information on the shelf life can be found on the public summary of the Australian Register of Therapeutic Goods (ARTG). The expiry date can be found on the packaging.
6.4 Special Precautions for Storage
Store below 30°C. Do not freeze.
Exposure to the heat should be minimised. Avoid excessive heat.
6.5 Nature and Contents of Container
Baxter Potassium Chloride and Sodium Chloride IV Infusion is supplied in Viaflex plastic containers as a single unit dose (see Section 2 Qualitative and Quantitative Composition, Table 1).
6.6 Special Precautions for Disposal
Any unused product or waste material should be disposed of in accordance with local requirements.
Summary Table of Changes


 For the full list of excipients, see Section 6.1 List of Excipients.
For the full list of excipients, see Section 6.1 List of Excipients.