What in this leaflet
Read this leaflet carefully before you start to use Betoptic.
This leaflet answers some common questions about Betoptic. It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
The information in this leaflet was last updated on the date listed on the final page. More recent information on the medicine may be available.
You should ensure that you speak to your pharmacist or doctor to obtain the most up to date information on the medicine.
You can also download the most up to date leaflet from www.novartis.com.au
The updates may contain important information about the medicine and its use of which you should be aware.
All medicines have risks and benefits. Your doctor has weighed the risks of you using Betoptic against the benefits they expect it will have for you.
If you have any concerns about using this medicine, ask your doctor or pharmacist.
Keep this leaflet with your medicine.
You may need to read it again.
What BETOPTIC is used for
Betoptic contains the active ingredient betaxolol hydrochloride. Betaxolol hydrochloride belongs to a class of medicines known as "beta-adrenergic blocking agents".
Your doctor has prescribed Betoptic for you because the pressure within your eye(s), known as "intraocular pressure" is higher than normal. This raised pressure may damage your eyesight and lead to a condition known as glaucoma.
There are usually no symptoms of glaucoma. If glaucoma is not treated it can lead to serious problems, including total blindness. Untreated glaucoma is one of the most common causes of blindness.
Betoptic is used, either alone or in combination with other medicines, to lower the raised pressure within your eye(s). Betoptic does this by reducing the amount of fluid produced within your eye(s).
Although Betoptic helps control your glaucoma it does not cure it. So you must keep using it until your doctor tells you to stop.
For more information about glaucoma, contact Glaucoma Australia Inc. (https://glaucoma.org.au/home).
Ask your doctor if you have any questions about why Betoptic has been prescribed for you. Your doctor may have prescribed it for another reason.
Use in Children
Betoptic is not recommended for use in children. The safety and effectiveness of Betoptic in children has not been established.
Before you use BETOPTIC
When you must not use it
Let your doctor know if any of the following applies to you before you start using Betoptic:
- You are allergic to betaxolol hydrochloride or to any of the other ingredients in these eye drops that are listed under "Product Description".
Some of the symptoms of an allergic reaction may include:
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body
- rash, itching or hives on the skin.
Tell your doctor if:
- You have a very slow heartbeat or irregular heartbeat
- You have cardiac failure or any other serious heart conditions.
If you have not told your doctor about any of the above, tell him/her before you start using Betoptic.
Do not use this medicine if the expiry date has passed, the packaging is torn or the safety seal around the closure and neck area is broken. If it has expired or is damaged, return it to your pharmacist for disposal.
If you use your medicine after the expiry date has passed, it may not work as well.
Before you start to use it
Tell your doctor if:
-
You are pregnant, or intend to become pregnant
Your doctor will discuss the possible risks and benefits of using Betoptic during pregnancy. -
You are breast-feeding or intend to breast-feed
Your doctor will discuss the possible risks and benefits of using Betoptic when you are breast-feeding.
Tell your doctor if have or have had any of the following medical conditions:
- Any type of respiratory or breathing disorder (e.g. wheezing or asthma)
- Diabetes
- An overactive thyroid gland
- Any form of muscle weakness
- Heart failure or heart block or any heart condition
- Severe circulation disorders e.g. Raynaud's disease or syndrome
- A condition called phaeochromocytoma which is a tumour in the brain
- Metabolic acidosis where the body produces excessive acid that is not cleared by the kidneys.
Tell your doctor if you are about to have either major surgery or eye surgery. This includes those doctors treating you in hospital or in a clinic. Your dose of Betoptic may need to be adjusted or gradually stopped prior to surgery.
Tell your doctor if you are a contact lenses wearer. This is particularly important if you are currently using any other type of anti-glaucoma medication or any other eye drops.
Do not put Betoptic into your eye(s) while you are wearing soft contact lenses.
The preservative in Betoptic, benzalkonium chloride, may be deposited in contact lenses.
You can put your soft contact lenses back into your eyes 15 minutes after you have used Betoptic.
If you are not sure if you should start using Betoptic, talk to your doctor.
Taking or using other medicines
Tell your doctor or pharmacist if you are taking or using other medicines, including medicines that you get without a doctor's prescription from a pharmacy, supermarket or health food shop.
Some medicines and Betoptic interfere with each other. These include:
- other beta-blockers, calcium blockers, including amiodarone, and digitalis glycosides
- certain medicines used to treat lower blood pressure e.g. reserpine
- some medicines used to treat major mental illnesses.
These medicines may be affected by Betoptic or may affect how well it works. You may need different amounts of your medicines.
Your doctor or pharmacist have more information on medicines to be careful with or avoid while using this medicine.
How to use BETOPTIC
Follow all directions given to you by your doctor or pharmacist carefully. They may differ from the information contained in this leaflet.
If you do not understand the instructions on the box, ask your doctor or pharmacist for help.
How much to use
It is important that you shake Betoptic Eye Drops well before using them.
The usual dose of Betoptic Eye Drops is one drop in the affected eye(s) two times each day. Your dosing instructions will be printed on the label your pharmacist puts on the bottle or carton.
Using your eye drops at the same time each day will have the best effect on your eye pressure. It will also help you remember when to use the eye drops.
Do not use Betoptic more often than your doctor or pharmacist has told you.
If you have been using any other eye drops for the treatment of raised intraocular pressure or glaucoma, it may take several days to change from the old drops to Betoptic Eye Drops. It is important that you carefully follow your doctor's instructions for the changeover.
After using Betoptic wait at least 5 minutes before putting any other eye drops in your eye(s).
How to use it
Follow these steps to use Betoptic:
- Wash your hands thoroughly.
- Immediately before using a bottle for the first time, break the safety seal around the neck area and throw the loose plastic ring away.
- Shake the bottle well.
- Remove the cap from the bottle.
- Hold the bottle upside down in one hand between your thumb and middle finger (see Diagram 1).
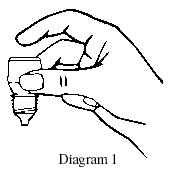
- While tilting your head back, gently pull the lower eyelid of your eye down using the forefinger of your other hand.
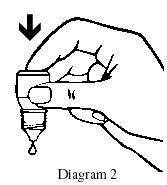
- Place the dropper tip close to, but not touching, your lower eyelid and gently tap or press the base of the bottle with your forefinger to release one drop (see Diagrams 2 and 3).
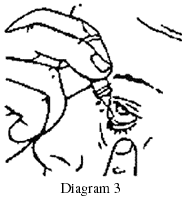
- Close your eye gently without blinking and press on the inside corner of your eye with the pad of your index finger for two minutes.
- If necessary, repeat the above steps for your other eye.
- Place the cap on the bottle and close it tightly.
- Wash your hands again.
You may feel a slight burning sensation in the eye shortly after using Betoptic. If this persists, or is very uncomfortable, contact your doctor or pharmacist.
Do not touch the tip of the dropper to your eye or to any other surface. This will help to prevent your eye drops becoming dirty or contaminated.
If you wear contact lenses take the lenses out before you use Betoptic. Wait 15 minutes before putting back your contact lenses.
How long to use it
Continue using Betoptic every day as long as your doctor prescribes.
Betoptic helps control your condition but does not cure it.
If you forget to use it
If you forget to use Betoptic, you should put the drops that you missed in as soon as you remember and then go back to using them normally. If it is almost time for your next dose, skip the dose that you have missed and take your next dose when you are due to.
Never use a double dose to make up for the one that you missed.
If you are not sure what to do, contact your doctor or pharmacist.
If you use too much (overdose)
If you accidentally put too many drops in your eye(s), immediately rinse your eye(s) with warm water or normal saline.
If anyone accidentally swallows Betoptic Eye Drops, immediately telephone your doctor, the Poisons Information Centre on 13 11 26 766 or go to Accident and Emergency at the nearest hospital. Do this even if there are no signs of discomfort or poisoning.
While you are using BETOPTIC
Things you must do
To make sure that Betoptic is working properly, have your eye pressure checked regularly by your doctor.
Have your eyes checked for any other changes you experience.
Tell your doctor if you become pregnant while you are using Betoptic.
Tell your doctor and pharmacist that you are using Betoptic before you start taking or using any other medicines.
Things that you must not do
Do not let children handle Betoptic Eye Drops. If a child accidentally swallows any of the drops read the instructions under "If you use too much (overdose)".
Do not give this medicine to anyone else, even if they appear to have the same condition as you.
Things to be careful of
Be careful driving or operation machinery until you know how Betoptic affects you. As with any eye medicines, temporary blurred vision or other visual disturbances may affect the ability to drive and use machinery in some people. If blurred vision occurs when you use your drops, wait until your vision is clear before driving.
Side Effects
All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical treatment if you get some of the side effects.
Tell your doctor as soon as possible if you do not feel well while you are using Betoptic.
Most side effects from Betoptic occur in, or around, the eye.
These include:
- Discomfort or pain in the eye(s)
- A loss of feeling to the surface of the eye(s)
- Redness, inflammation, irritation and/or itching in your eye(s), eyelids or the surrounding lining of the eyelids
- Inflammation of the cornea (clear front portion of your eye) (punctuate keratitis)
- Blurred vision and/or problems seeing clearly
- A feeling that something is in your eye(s)
- Eyelid spasms
- A dry eye(s)
- Crusty eyelash(es) or eyelids
- Discomfort in the eye(s) due to a greater sensitivity to light
- Weakness or easily fatigued eyes.
Occasionally, some people notice unwanted effects in the rest of their body as a result of using Betoptic.
Tell your doctor immediately if you notice any of the following effects:
- Changes in breathing (e.g. wheezing or asthma)
- Cough
- Respiratory infection, sinusitis, runny nose
- Circulation problems
- Fast or slowing of heart beat, irregular heart beat
- Nausea
- Trouble sleeping
- Headache
- Dizziness
- Fainting
- Tiredness and/or depression
- Decreased libido
- Hives and more severe forms of skin rash
- Flaking skin and/or hair loss
- Sore tongue
- Altered taste sensation.
These may be serious side effects. You may need urgent medical attention. Serious side effects are rare.
Other side effects not listed above may occur in some people.
After using BETOPTIC
Keep Betoptic Eye Drops in a cool place, below 25°C. Do not freeze.
Store the bottle in the outer carton.
Do not leave Betoptic Eye Drops in the bathroom or near a sink.
Do not leave it on a window sill or in the car. Heat and dampness can destroy some medicines.
Keep Betoptic Eye Drops, and all other medicines, in a safe place away from the sight or reach of children. A locked cupboard at least one-and-a half metres above the ground is a good place to store medicines.
Discard each bottle of Betoptic Eye Drops 4 weeks after it has been opened. Write the date the bottle was opened on the label to remind you when to discard the bottle.
Product Description
What it looks like
Betoptic Eye drops are a sterile isotonic solution supplied in a 5 mL dispensing bottle.
Ingredients
The active ingredient in Betoptic Eye Drops is betaxolol hydrochloride equivalent to betaxolol 5mg/mL.
Betoptic Eye Drops also contain:
- Benzalkonium chloride (as a preservative)
- Disodium edetate
- Sodium chloride
- Purified water.
Supplier
This product is supplied in Australia by:
Novartis Pharmaceuticals Australia Pty Limited
ABN 18 004 244 160
54 Waterloo Road
Macquarie Park NSW 2113
Telephone No. 1800 671 203
www.novartis.com.au
Australian Registration Number
Betoptic Eye Drops: Aust R No. 25272
Date of Preparation
This leaflet was revised in October 2023.
® Registered Trademark.
© Novartis Pharmaceuticals Australia Pty Limited
Internal document code:
(bet271023c) based on PI (bet271023i)
Published by MIMS December 2023
