What is in this leaflet
This leaflet answers some common questions about Biostate®. It does not contain complete information about Biostate®.
It does not take the place of talking to your doctor.
If you have any concerns about using this medicine, please talk to your doctor. Follow your doctor’s advice even if it is different from what this leaflet says.
Please read this leaflet carefully and keep it for future reference.
The information in this leaflet is subject to change. Please check with your Haemophilia Treatment Centre if there is any new information about this medicine that you should know since you were last treated.
What Biostate® is used for
Biostate® contains FVIII and VWF in a purified and concentrated form. Both FVIII and VWF are blood proteins that are essential for normal blood clotting.
Biostate® is used in patients with von Willebrand Disease (VWD), a bleeding disorder resulting from low levels of VWF or abnormal VWF. Individuals with VWD tend to take longer than normal to form blood clots, and tend to bleed from the skin and mucous membranes such as the nose, mouth and intestines. Because VWF provides stability for the fragile FVIII protein in the blood, patients with VWD may also have low FVIII levels.
Biostate® is also used in patients with haemophilia A, a bleeding disorder, in which there are low levels of FVIII or abnormal FVIII. Individuals with low levels or abnormal FVIII have difficulty in forming blood clots, with these clots often taking longer than normal to be made. Sometimes the individual may bleed unexpectedly into their joints, muscles or internal organs.
Ask your Haemophilia Treatment Centre if you have any questions about why Biostate® has been prescribed for you.
Before you are given Biostate®
Tell your doctor if you:
- have allergies to any medicines
- are taking or using any other medicines. These include medicines bought from pharmacies, supermarkets and health food stores.
- have any other medical conditions
- are pregnant or breast-feeding
- become pregnant during your treatment.
If you want further information, consult your doctor or Haemophilia Treatment Centre.
About blood products
Biostate® is manufactured from human plasma (the liquid component of blood) collected by Australian Red Cross Lifeblood. When products are made from human blood and injected into you, it is possible that viruses or other substances could be present in the product and cause an illness. These could be viruses such as hepatitis, human immunodeficiency virus (HIV), or parvovirus B19 and theoretically the Creutzfeldt-Jakob Disease (CJD) agent. There could also be other infectious agents some of which may not yet have been discovered.
To reduce the risk of this happening, strict controls are applied when selecting blood donors and donations. In addition, extra steps are taken when manufacturing this product. Biostate® is specially treated to remove and kill viruses. This special treatment is considered effective against certain viruses known as enveloped viruses (such as HIV and hepatitis B and C), and also the non-enveloped virus, hepatitis A. They may be of limited value against non-enveloped viruses such as parvovirus B19. Despite these safety measures, such products may still potentially transmit disease.
Vaccines are available against some of these viruses and your doctor will be able to help you decide whether it is worthwhile having any of those vaccines.
Please discuss the risks and benefits of Biostate® with your doctor.
How to use Biostate®
The dosage and administration of Biostate® must be carefully controlled. Your doctor will be responsible for determining what dose is appropriate to your condition.
Biostate® will usually be given in a hospital. Should your doctor decide that treatment at home is appropriate, your Haemophilia Treatment Centre will provide detailed instructions on how to use Biostate®.
The following procedures are given as a guide only:
Preparing Biostate® for administration
You will need one 5 mL Water for Injections vial for each 250 IU and 500 IU (100 IU/mL) vial of Biostate®, or one 10 mL Water for Injections vial for each 500 IU (50 IU/mL) and 1000 IU vial of Biostate®.
- Ensure you have all the required equipment to administer Biostate®.
- Allow the vials of Biostate® and Water for Injections to reach room temperature prior to use, which may take up to one hour. Do not warm the Water for Injections in hot water.
- Remove jewellery, watches, rings, etc.
- Wash hands with soap and water, dry with a clean towel.
- Select an appropriate work area with good lighting and a surface which can be cleaned (such as a kitchen table).
- Using a clean cloth or paper towel, clean the preparation area with methylated spirits.
- Gather the equipment to be used.
Equipment
- One carton of Biostate® containing:
- one vial of Biostate®
- one vial of Water for Injections
- one Mix2Vial™ filter transfer set.
Check the expiry date of each item. Do not use if expired. - two alcohol wipes
- sharps container
- waste container for discarding biological material
- plastic syringe(s)
- adhesive tape
- cotton balls
- intravenous injection set
- gloves.
Instructions for Biostate® reconstitution
Follow these steps to prepare the injection.
- Wash hands with soap and water, dry with a clean towel.
- Ensure Biostate® and Water for Injections are at room temperature.
- Remove flip top caps from Biostate® and Water for Injections vials.
- Wipe the rubber stoppers of both the Biostate® and Water for Injections vials with the alcohol wipes and allow to dry for two minutes. Do not leave the alcohol wipes resting on the stoppers. Do not touch the rubber stoppers with your fingers.
- Open the lid of the Mix2Vial™ packaging. If the seal of the lid is not intact or you have any other concerns about the integrity of the Mix2Vial™, do not use it but return it to your Haemophilia Treatment Centre or Australian Red Cross Lifeblood. Place the Water for Injections vial on a level surface and hold the vial firmly. Take the Mix2Vial™ together with the outer package, invert it and push the blue plastic cannula of the Mix2Vial™ firmly through the rubber stopper of the Water for Injections vial. See Figure 1, (5 mL Water for Injections vial is provided for 250 IU vial and 500 IU (100 IU/mL) vial and 10 mL Water for Injections vial is provided for 500 IU (50 IU/mL) vial and 1000 IU vial).
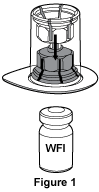
WFI = Water for Injections
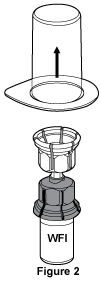
- While holding onto the vial of Water for Injections, carefully remove the outer package from the Mix2Vial™, being careful to leave the Mix2Vial™ firmly attached to the vial of Water for Injections, see Figure 2. Make sure that you only remove the outer package and not the Mix2Vial™.
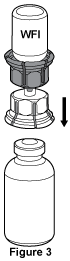
- With the Biostate® vial held firmly on a level surface, invert the Water for Injections with the Mix2Vial™ attached and push the transparent plastic cannula end of the Mix2Vial™ firmly through the stopper of the Biostate® vial, see Figure 3. The water will be drawn into the vial by the vacuum contained within the Biostate® vial. If water is not drawn into the vial, it means that there is no vacuum in the vial and the seal may be faulty. Do not use the product but return it to your Haemophilia Treatment Centre or Australian Red Cross Lifeblood.
Note: The Mix2Vial™ is intended to filter the contents of a single vial of Biostate® only. If multiple vials of Biostate® are to be given, a separate Mix2Vial™ must be used for each vial. - With the Water for Injections and Biostate® vials still attached to the Mix2Vial™, gently swirl (do not shake) the Biostate® vial until all of the product is dissolved. Ensure the contents of the vial are completely dissolved. If a clot or gel forms do not use the product but return it to your Haemophilia Treatment Centre or Australian Red Cross Lifeblood.
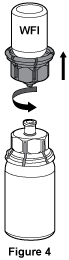
- Once the contents of the Biostate® vial are completely dissolved, firmly hold both the transparent and blue parts of the Mix2Vial™. Unscrew the Mix2Vial™ into two separate pieces, see Figure 4. Discard the empty Water for Injections vial with the blue part of the Mix2Vial™ still attached into an appropriate waste container.
- While the Biostate® vial is upright, attach a plastic disposable syringe to the transparent part of the Mix2Vial™. Invert the system and draw the Biostate® into the syringe by pulling the plunger back slowly.
- Once the Biostate® has been transferred into the syringe, firmly hold the barrel of the syringe (keeping the syringe plunger facing down) and detach the Mix2Vial™ from the syringe. Do not use the Mix2Vial™ for injection.
Note: One large syringe may be used to withdraw Biostate® from multiple vials. - Discard the empty vial of Biostate® with the transparent part of the Mix2Vial™ attached, into an appropriate waste container.
Use Biostate® as soon as you can after preparation. (Make sure it is used for one person on one occasion only). The solution must not be stored for longer than 8 hours and the infusion should be completed as soon as practicable, as Biostate® does not contain an antimicrobial preservative. Any unused portion remaining in the vial must be discarded appropriately.
Do not refrigerate Biostate® once it has been prepared.
Injection administration procedure guidelines
The injection is best given by following each of the steps outlined in turn.
Should any of the symptoms listed under ‘Side effects’ develop, stop the infusion immediately and contact your Haemophilia Treatment Centre.
- Apply tourniquet. Select injection site.
- Wash hands with soap and water, dry with a clean towel.
- Cleanse the skin area with an alcohol wipe, allow to dry.
- Put on gloves.
- Insert intravenous needle into vein.
- Secure needle with adhesive tape.
- Attach the syringe containing Biostate® to the intravenous needle.
- Gently pull back the plunger until the tubing is filled with blood.
- Release the tourniquet.
- Administer the Biostate® solution slowly (usually within 5 minutes, or as tolerated).
- Carefully remove adhesive tape.
- Carefully remove the intravenous needle with syringe attached and place directly into the sharps container.
- Apply pressure to the injection site using a cotton ball for one to two minutes. Apply dressing if necessary.
- Discard all used sharps into the sharps container, and dispose of the other used equipment appropriately.
- Wash hands with soap and water, dry with a clean towel.
Side effects
Along with their intended effects, medicines may cause some unwanted effects, which can sometimes be serious. Furthermore, individual patients may react differently to the same dose of the same medicine. This applies to Biostate®, although severe reactions after Biostate® injection are rare. Ask your Haemophilia Treatment Centre if you need more information.
Stop using this product immediately and contact your doctor if any of the following side effects occur:
- headache
- pain (such as back pain, joint pains and bone pains)
- dizziness
- anxiety
- reddening of the face or neck
- chest pain
- sweating
- feeling sick or vomiting
- strange taste in the mouth
- irritation of the vein used for infusion (which may include swelling, redness or tenderness)
- shortness of breath or difficulty breathing.
Contact your doctor immediately if you experience any of these symptoms at any time:
- fever
- loss of appetite
- extreme tiredness
- abdominal pain
- jaundice (yellow skin and eyes)
- dark urine
- joint pains
- skin rashes.
Inhibitors
Treatment with factor VIII products such as Biostate® may sometimes lead to the formation of antibodies (inhibitors) which neutralise factor VIII and reduce the effectiveness of the treatment. Your doctor will monitor you for development of these inhibitors and if they suspect that an inhibitor is present, the level of inhibitor will be measured using the appropriate laboratory tests. In some patients, inhibitors can be successfully overcome with larger doses of factor VIII, but for some patients it may be necessary to switch to a different treatment.
Overdose
Cases of overdose have been observed. No severe adverse reactions were associated with these cases. The risk of thromboembolic events cannot be excluded in cases of major overdose, especially in patients with VWD. If you have any questions, consult your doctor.
Storing Biostate®
Store at 2°C to 8°C (Refrigerate. Do not freeze). Biostate® can be stored at 25°C or below for a single period of 6 months. The product must not be returned to refrigeration after storage at 25°C or below. Protect from light.
Do not use after the expiry date.
Further information
This is not all the information that is available on Biostate®. If you have any more questions or are not sure about anything, ask your Haemophilia Treatment Centre.
Product description
What it looks like
Biostate® is a white or pale yellow powder contained in a glass vial. Biostate® is registered in four presentations. Each pack size contains:
- 250 IU vial of Biostate® (with a FVIII concentration of 50 IU/mL and a VWF concentration of 120 IU/mL), 5 mL vial of Water for Injections, and a special filter transfer set called a Mix2Vial™.
- 500 IU vial of Biostate® (with a FVIII concentration of 50 IU/mL and a VWF concentration of 120 IU/mL), 10 mL vial of Water for Injections, and a special filter transfer set called a Mix2Vial™.
- 500 IU vial of Biostate®, (with a FVIII concentration of 100 IU/mL and a VWF concentration of 240 IU/mL), 5 mL vial of Water for Injections, and a special filter transfer set called a Mix2Vial™.
- 1000 IU vial of Biostate®, (with a FVIII concentration of 100 IU/mL and a VWF concentration of 240 IU/mL), 10 mL vial of Water for Injections, and a special filter transfer set called a Mix2Vial™.
Ingredients
Biostate® contains FVIII and VWF as the active ingredients. It also contains:
- sucrose
- sodium citrate dihydrate
- sodium chloride
- trometamol
- calcium chloride dihydrate
- human albumin
- human plasma proteins.
This product is packaged in latex free materials.
Manufacturer
Biostate® is manufactured in Australia by:
CSL Behring (Australia) Pty Ltd
ABN 48 160 734 761
189–209 Camp Road
Broadmeadows VIC 3047
Australia
Distributor
Australian Red Cross Lifeblood
Date of revision
August 2021
Australian Register Numbers
250 IU FVIII/600 IU VWF:
AUST R 73032
500 IU FVIII/1200 IU VWF:
AUST R 79993
500 IU FVIII/1200 IU VWF:
AUST R 150648
1000 IU FVIII/2400 IU VWF:
AUST R 150657
® Registered trademark of CSL Limited
™ Mix2Vial is a trademark of West Pharmaceutical Services, Inc. or a subsidiary thereof
Published by MIMS October 2021
