Brenzys®
| Consumer Medicine Information (CMI) summary |
The full CMI on the next page has more details. If you are worried about using this medicine, speak to your doctor or pharmacist.
| 1. Why am I using Brenzys®? |
Brenzys® contains the active ingredient etanercept. Brenzys® is used to treat specific types of joint inflammation and skin conditions, namely rheumatoid arthritis, ankylosing spondylitis, non-radiographic axial spondyloarthritis (nr-AxSpA), psoriatic arthritis and plaque psoriasis.
For more information, see Section 1. Why am I using Brenzys®? in the full CMI.
| 2. What should I know before I use Brenzys®? |
Do not use if you have ever had an allergic reaction to Brenzys® or any of the ingredients listed at the end of the CMI.
Talk to your doctor if you have any other medical conditions, take any other medicines, or are pregnant or plan to become pregnant or are breastfeeding.
For more information, see Section 2. What should I know before I use Brenzys®? in the full CMI.
| 3. What if I am taking other medicines? |
Some medicines may interfere with Brenzys® and affect how it works.
A list of these medicines is in Section 3. What if I am taking other medicines? in the full CMI.
| 4. How do I use Brenzys®? |
- Brenzys® is given in adults once a week as a 50 mg dose. Brenzys® is not indicated for use in children less than 18 years of age. Other etanercept products are available for use in children.
- Brenzys® is injected under the skin and is for single use in one patient only. Refer to Instructions for Use provided in the full CMI.
More instructions can be found in Section 4. How do I use Brenzys? in the full CMI.
| 5. What should I know while using Brenzys®? |
| Things you should do |
|
| Things you should not do |
|
| Looking after your medicine |
|
For more information, see Section 5. What should I know while using Brenzys®? in the full CMI.
| 6. Are there any side effects? |
Common side effects include a mild reaction in the area of injection, infections and inflammation, allergic reactions, rash, itching, fever, headache and raised liver enzymes.
For more information, including what to do if you have any side effects, see Section 6. Are there any side effects? in the full CMI.
Brenzys®
Active ingredient(s): etanercept
| Consumer Medicine Information (CMI) |
This leaflet provides important information about using Brenzys®. You should also speak to your doctor or pharmacist if you would like further information or if you have any concerns or questions about using Brenzys®.
Where to find information in this leaflet:
1. Why am I using Brenzys®?
2. What should I know before I use Brenzys®?
3. What if I am taking other medicines?
4. How do I use Brenzys®?
5. What should I know while using Brenzys®?
6. Are there any side effects?
7. Product details
| 1. Why am I using Brenzys®? |
Brenzys® contains the active ingredient etanercept.
Etanercept is a biotechnology-derived protein that works by binding to and inactivating Tumour Necrosis Factor (TNF), a naturally occurring chemical in your bloodstream that contributes to pain and swelling in the joints.
Brenzys® is used in the treatment of specific inflammatory joint or skin conditions by reducing the pain and swelling of rheumatoid arthritis and psoriasis, helping to treat the skin lesions of psoriasis and psoriatic arthritis, and improving the condition of patients with ankylosing spondylitis and nonradiographic axial spondyloarthritis (nr-AxSpA).
Your doctor may have prescribed Brenzys® for another reason. Ask your doctor if you have any questions about why Brenzys® has been prescribed for you.
| 2. What should I know before I use Brenzys®? |
Warnings
Do not use Brenzys® if:
- you are allergic to etanercept or any of the ingredients listed at the end of this leaflet.
- Always check the ingredients to make sure you can use this medicine.
- you have, or are at risk of developing, sepsis (blood poisoning), or long-term or localised infection (for example, leg ulcers).
Sepsis is a serious infection causing fever, headache, joint aches and pains, sore or weak muscles, and increased heart rate. Brenzys® can affect your body's ability to fight a serious infection.
If you are not sure whether you have a serious infection, check with your doctor. - you are currently taking anakinra or other similar medicines known as Interleukin-1 antagonists.
- the packaging is torn or shows signs of tampering
- the expiry date printed on the pack has passed.
If you use Brenzys® after the expiry date has passed, it may have no effect at all, or worse, have an entirely unexpected effect.
Check with your doctor if you:
- have any allergies to:
- any other medicines,
- any other substances, such as foods, preservatives or dyes. - have any other medical conditions, especially:
- serious infection including sepsis, tuberculosis or a history of recurring infections,
- low resistance to disease,
- diabetes,
- liver problems or hepatitis B or hepatitis C, viruses that affect the liver,
- heart failure,
- blood disorders,
- cancer,
- are about to have major surgery,
- nerve disorders including multiple sclerosis or optic neuritis (inflammation of the nerves of the eyes),
- seizures,
- chickenpox or have been recently exposed to chickenpox. - take any medicines for any other condition,
- you are pregnant or intend to become pregnant,
- you are breast-feeding or plan to breast-feed.
If you are not sure whether you should start taking this medicine, talk to your doctor.
During treatment, you may be at risk of developing certain side effects. It is important you understand these risks and how to monitor for them. See additional information under Section 6. Are there any side effects?
Pregnancy and breastfeeding
Check with your doctor if you are pregnant or intend to become pregnant.
The effects of Brenzys® in pregnant women are not well understood, therefore Brenzys® should only be used during pregnancy if clearly needed. If you become pregnant while using Brenzys®, contact your doctor. Your doctor will help you to decide whether the benefits of treatment outweigh the potential risk to your baby.
Talk to your doctor if you are breastfeeding or intend to breastfeed.
Brenzys® passes through breast milk. Therefore, if you are breast-feeding, you should discuss with your doctor whether to stop breastfeeding or stop using Brenzys®.
This medicine is available only with a doctor's prescription. Brenzys® is not addictive.
| 3. What if I am taking other medicines? |
Tell your doctor or pharmacist if you are taking any other medicines, including any medicines, vitamins or supplements that you buy without a prescription from your pharmacy, supermarket or health food shop.
Some medicines may interfere with Brenzys® and affect how it works.
Medicines that increase risk of side effects when used with Brenzys®:
- abatacept and Interleukin-1 antagonists such as anakinra. These medicines should not be used with Brenzys®.
- cyclophosphamide, a medicine used to treat cancer or prevent transplant rejection. Use with Brenzys® is not recommended.
- sulfasalazine.
Medicines that Brenzys® may interfere with:
- some vaccines
- warfarin, a medicine used to thin the blood and prevent blood clots
- digoxin, a medicine used to improve the strength and efficiency of the heart, or to control the rate and rhythm of the heartbeat
- medicines used to treat diabetes
Check with your doctor or pharmacist if you are not sure about what medicines, vitamins or supplements you are taking and if these affect Brenzys®.
| 4. How do I use Brenzys®? |
Your doctor will tell you how to inject Brenzys®. A different site should be used for each new injection. Each new injection should be given at least 3 cm from an old site.
How much to use
Adults
- The recommended dose for adults is 50 mg per week, injected under the skin. Your doctor may determine a different frequency at which to inject Brenzys®.
- If you are being treated for psoriasis, your doctor may prescribe a higher dose of Brenzys® when you first begin your treatment.
- If you are being treated for nr-AxSpA and Brenzys® has no effect on your condition within 12 weeks, your doctor may tell you to stop using this medicine.
- Follow the instructions provided and use Brenzys® until your doctor tells you to stop.
Children
Brenzys® is not indicated for use in children less than 18 years of age. Other etanercept products are available for use in children.
When to use Brenzys®
- Brenzys® should be used at the same time each week as directed by your doctor.
How to use Brenzys®
Your doctor will supervise and advise you on how to use Brenzys® when you first start. If your doctor thinks it is appropriate, they can train you on how to self-inject Brenzys®.
Brenzys® should be administered according to the instructions provided in the Instructions for Use leaflet included at the end of this document.
Solution for injection only:
- After allowing the Brenzys® solution to reach room temperature (approximately 15 to 30 minutes), immediate use is recommended.
- Each syringe or Auto-injector of Brenzys® is for single use only, in one patient only. Discard any residue.
Follow all directions given to you by your doctor or pharmacist carefully.
They may differ from the information contained in this leaflet.
If you are injecting Brenzys® yourself, you must follow the detailed instructions provided in this leaflet.
Brenzys® is injected under the skin. When using the syringes or Auto-injector, it is important that you do not pull back on the plunger. Brenzys® can be injected by your doctor, nurse, carer or by yourself.
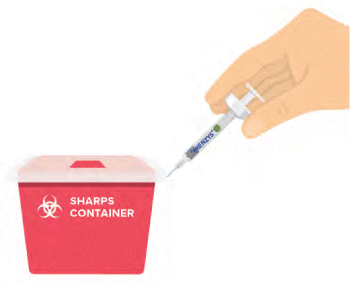
When you have finished injecting Brenzys®, discard the syringe or Auto-injector into a sharps container.
If you do not understand the instructions for injecting Brenzys® found in the carton, ask your doctor or pharmacist for help.
To help you remember, use a diary to write in the days of the week you should have a Brenzys® injection.
You should continue to inject Brenzys® for as long as your doctor recommends.
Never inject more than the dose recommended by your doctor.
If you feel that the effect of Brenzys® is too strong or too weak, talk to your doctor or pharmacist.
If you forget to use Brenzys®
Brenzys® should be used regularly at the same time each week as agreed with your doctor. If you miss your dose at the usual time, you should inject the next dose as soon as you remember if it is within 48 hours since the scheduled dose time.
If it is almost time for your next dose i.e. within 48 hours of your next dose, skip the dose you missed and take your next dose when you are meant to.
Do not take a double dose to make up for the dose you missed.
If you use too much Brenzys®
If you think that you have used too much Brenzys®, you may need urgent medical attention.
You should immediately:
- phone the Poisons Information Centre
(by calling 13 11 26), or - contact your doctor, or
- go to the Emergency Department at your nearest hospital.
You should do this even if there are no signs of discomfort or poisoning.
Always take the labelled medicine carton with you, even if it is empty.
You may need urgent medical attention. There is very limited data on overdose with Brenzys®. Ask your doctor if you have any concerns.
| 5. What should I know while using Brenzys®? |
Things you should do
Tell any other doctors, dentists and pharmacists who are treating you that you are using Brenzys®.
Tell your doctor if Brenzys® is not making your condition better.
Keep a record of the Lot number of each Brenzys® pack that you use.
Call your doctor straight away if you:
- Have or develop any serious infection while using Brenzys®. Do not inject any more Brenzys® and contact your doctor immediately.
- Have any symptoms such as persistent fever, sore throat, bruising, bleeding or paleness.
These symptoms may point to the existence of a potentially life-threatening blood disorder, which may require you to stop taking Brenzys®.
Remind any doctor or dentist you visit that you are using Brenzys®.
Things you should not do
- Do not stop using this medicine suddenly or lower the dosage without checking with your doctor.
- Do not shake the Brenzys® syringe or Auto-injector.
- Do not stop using Brenzys® because you are feeling better, unless your doctor advises you to. Your condition may flare up if you reduce the dose or stop using Brenzys®.
- Do not give Brenzys® to anyone else even if they have the same condition as you.
- Do not use Brenzys® to treat any other complaints unless your doctor tells you to.
- Do not stop using Brenzys®, or lower the dosage, without checking with your doctor.
Driving or using machines
It is not known whether Brenzys® causes dizziness or drowsiness.
Drinking alcohol
No information is available.
Looking after your medicine
Follow the instructions in the carton on how to take care of your medicine properly.
Keep Brenzys® in a refrigerator where the temperature stays between 2°C and 8°C.
Keep it away from moisture, heat or sunlight; for example, do not store it:
- in the bathroom or near a sink, or in the car or on window sills.
Store Brenzys® pre-filled syringes and auto-injectors in their cartons to protect them from light.
Heat and dampness can destroy some medicines.
If it is not possible to store Brenzys® in the refrigerator, it may be stored out of the refrigerator (below 25°C) for up to 4 weeks (e.g. when travelling).
If you have stored Brenzys® at room temperature for any period of time (even if returned to the refrigerator) you must use it within 4 weeks from the time you first took it out of the refrigerator, or else you must discard it.
Do not use Brenzys® if it has been exposed to high temperatures or has been out of the refrigerator for more than 4 weeks.
Do not use this medicine after the expiry date.
Keep it where young children cannot reach it.
Getting rid of any unwanted medicine
If you no longer need to use this medicine or it is out of date, take it to any pharmacy for safe disposal.
| 6. Are there any side effects? |
All medicines can have side effects. If you do experience any side effects, most of them are minor and temporary. However, some side effects may need medical attention.
See the information below and, if you need to, ask your doctor or pharmacist if you have any further questions about side effects.
Less serious side effects
| Less serious side effects | What to do |
Injection site reactions:
Symptoms of an allergic reaction may include:
| Speak to your doctor if you have any of these common side effects and they worry you. |
Serious side effects
| Serious side effects | What to do |
Infections:
| Call your doctor straight away, or go straight to the Emergency Department at your nearest hospital if you notice any of these serious side effects. |
There have been reports of some types of cancer developing in patients using Brenzys® and other TNF blocking medicines.
These include skin cancers, cancers that affect the lymph system called lymphoma and Kaposi's sarcoma (which also affects the organs, skin, mouth, nose or throat), or affect the blood system called leukaemia. The role of Brenzys® in the development of cancer is not known.
Tell your doctor or pharmacist if you notice anything else that may be making you feel unwell.
Other side effects not listed here may occur in some people.
Reporting side effects
After you have received medical advice for any side effects you experience, you can report side effects to the Therapeutic Goods Administration online at www.tga.gov.au/reporting-problems. By reporting side effects, you can help provide more information on the safety of this medicine.
Always make sure you speak to your doctor or pharmacist before you decide to stop taking any of your medicines.
| 7. Product details |
This medicine is only available with a doctor's prescription.
Solution for injection
Pre-filled syringe, Auto-injector
| Active ingredient (main ingredient) | Etanercept |
| Other ingredients (inactive ingredients) | Sucrose Sodium chloride Sodium phosphate monobasic monohydrate Sodium phosphate dibasic (heptahydrate) Water |
Do not take this medicine if you are allergic to any of these ingredients.
What Brenzys® looks like
Pre-filled Syringe (Solution for injection):
Brenzys® Pre-filled Syringe is supplied in a kit containing:
- Four single-dose pre-filled glass syringes containing Brenzys® solution.
Each syringe contains 50 mg of etanercept in 1 mL of Brenzys® solution.
(AUST R 245252)
Auto-injector (Solution for injection)
Brenzys® Auto-injector is supplied in a kit containing:
- Either one or four single-dose pre-filled glass syringes, each housed in a plastic Auto-injector.
Each syringe contains 50 mg of etanercept in 1 mL of Brenzys® solution.
- Two or four alcohol swabs are also provided in the kit.
(AUST R 245253)
Who distributes Brenzys®
Brenzys® is supplied by:
Sponsor:
SAMSUNG BIOEPIS AU PTY LTD
Level 16, 201 Elizabeth Street,
Sydney NSW 2000, Australia
Distributor:
Organon Pharma Pty Limited
Building A, 26 Talavera Road, Macquarie Park, NSW 2113,
Australia
This leaflet was prepared in October 2023.
Version 15.0
| Instructions for Use |
The following instructions are for preparing and giving a dose of BRENZYS® using a single-use pre-filled syringe.
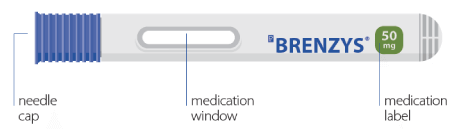
YOUR BRENZYS® PRE-FILLED SYRINGE
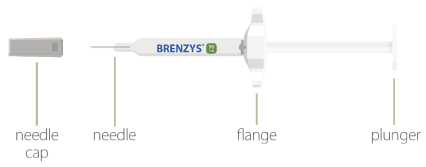
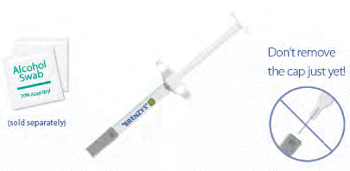
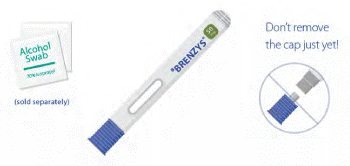
STEP 1: GATHER SUPPLIES
- Place your syringe and upopened alcohol swabs on a clean, dry surface.
- Remember to wash your hands!
- Don't remove the cap just yet!
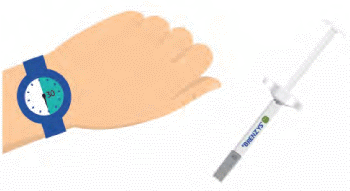
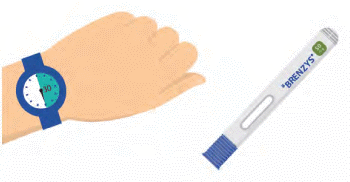
Step 2: WAIT 30 MINUTES
- Wait approximately 30 minutes for your syringe to warm-up to room temperature, which helps reduce your pain during injection.
- Don't remove the cap just yet!
Step 3: INSPECT MEDICINE & DATE
- Always make sure your medicine hasn't expired.
- The medicine should be clear or slightly opalescent, colourless or pale yellow, and may contain small white or almost transparent particles of protein.
- You may see an air bubble, and that's okay.
- Don't remove the cap just yet!
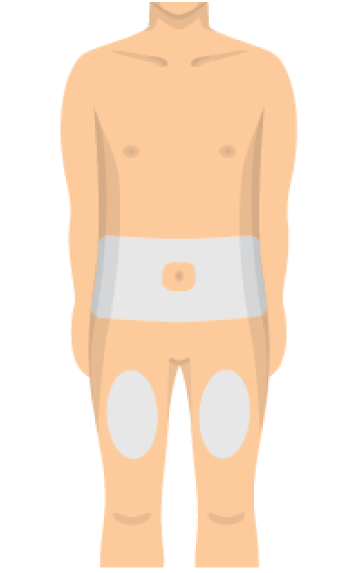
Step 4: CHOOSE SITE & CLEAN SKIN
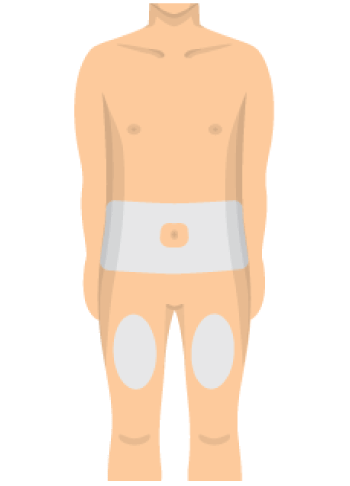
- Choose an injection site on your body.
- Your abdomen or thighs are best.
- Wipe your skin at the injection site with an alcohol swab.
- Avoid skin that's sore, bruised, scarred, scaly or has red patches.
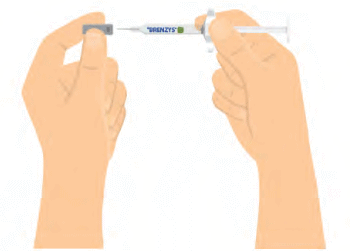
Step 5: REMOVE SYRINGE CAP
- Carefully remove the needle cap.
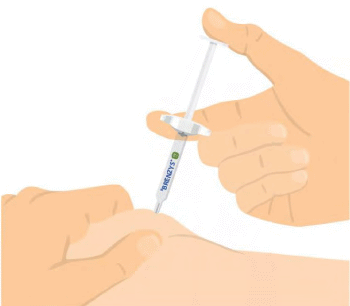
Step 6: PINCH SKIN & INSERT NEEDLE
- Gently pinch your skin, and carefully insert the needle.
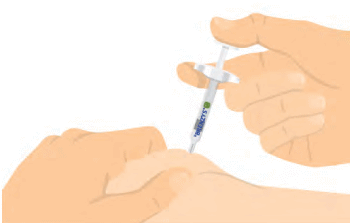
Step 7: PUSH PLUNGER ALL THE WAY
- Hold the syringe steady and press the plunger all the way down.
Step 8: REMOVE SYRINGE & DISPOSE
- Pull the syringe away from your skin and dispose of it in a sharps container.
- Don't recap or reuse your needle.
| Instructions for Use |
The following instructions are for preparing and giving a dose of BRENZYS® using a single-use auto-injector.
YOUR BRENZYS® AUTO-INJECTOR
Step 1: GATHER SUPPLIES
- Place your auto-injector and unopened alcohol swabs on a clean, dry surface.
- Remember to wash your hands!
- Don't remove the cap just yet!
Step 2: WAIT 30 MINUTES
- Wait approximately 30 minutes for your auto-injector to warm-up to room temperature, which helps reduce your pain during injection.
- Don't remove the cap just yet!
Step 3: INSPECT MEDICINE & DATE
- Always make sure your medicine hasn't expired.
- The medicine should be clear or slightly opalescent, colourless or pale yellow, and may contain small white or almost transparent particles of protein.
- You may see an air bubble, and that's okay.
- Don't remove the cap just yet!

Step 4: CHOOSE SITE & CLEAN SKIN
- Choose an injection site on your body.
- Your abdomen or thighs are best.
- Wipe your skin at the injection site with an alcohol swab.
- Avoid skin that's sore, bruised, scarred, scaly or has red patches.
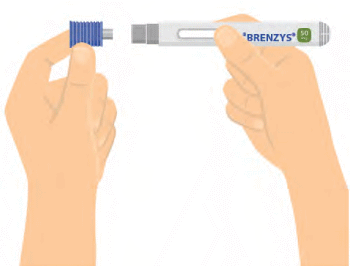
Step 5: REMOVE THE BLUE NEEDLE CAP
- Carefully remove the blue needle cap with a metal center from the auto-injector.
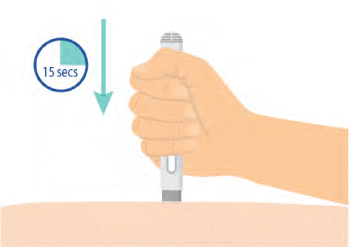
Step 6: PLACE GRAY NEEDLE SHIELD, PRESS DOWN, AND HOLD 15 SECONDS
- Place the gray needle shield straight on your skin, and push the entire auto-injector down firmly to start the injection.
- When you push down, the injection starts.
- You may hear a click.
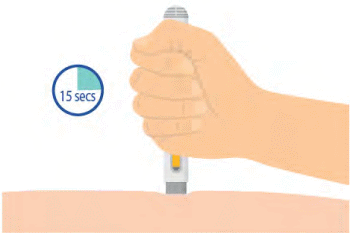
Step 7: AFTER 15 SECONDS, REMOVE AUTO-INJECTOR
- Hold the auto-injector against your skin.
- After 15 seconds, remove the auto-injector from the injection site.
- The medication window will change to yellow once the injection is completed.
- You may hear a second click.
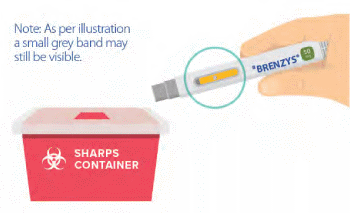
Step 8: CONFIRM COMPLETION & DISPOSE AUTO-INJECTOR
- Confirm that the medication window is yellow.
- Discard your auto-injector in a sharps container.
- As per illustration a small grey band may still be visible.
- If the window isn't yellow, you may not have received your full dose.
Published by MIMS December 2023
