What is in this leaflet
This leaflet answers some common questions about BRIMICA GENUAIR 340/12.
It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your pharmacist or doctor will be able to advise you about the risks and benefits of using BRIMICA GENUAIR 340/12.
If you have any concerns about using this medicine, ask your pharmacist or doctor.
Keep this leaflet with the medicine.
You may need to read it again.
What BRIMICA GENUAIR 340/12 is used for
BRIMICA GENUAIR 340/12 contains two different medicines called aclidinium bromide and formoterol fumarate dihydrate.
Both belong to a group of medicines known as bronchodilators. Bronchodilators relax airways and help keep bronchioles open.
BRIMICA GENUAIR 340/12 is a dry powder inhaler that uses your breath to deliver the medicine directly into your lungs. This makes it easier for chronic obstructive pulmonary disease (COPD) patients to breathe.
BRIMICA GENUAIR 340/12 is used to help open the airways and relieve symptoms of COPD, a serious long-term lung disease characterised by breathing difficulties. Regular use of BRIMICA GENUAIR 340/12 can help you when you have ongoing shortness of breath related to your disease and will help you to minimise the effects of the disease on your everyday life.
BRIMICA GENUAIR 340/12 is indicated for maintenance treatment of your COPD; it should not be used to treat a sudden attack of breathlessness or wheezing. If your COPD symptoms (breathlessness, wheezing, cough) do not improve or get worse you should contact your doctor for advice as soon as possible.
Ask your doctor if you have any questions about why BRIMICA GENUAIR 340/12 has been prescribed for you.
This medicine is only available with a doctor’s prescription.
It is not addictive.
Before you use BRIMICA GENUAIR 340/12
When you must not use it
Do not use BRIMICA GENUAIR 340/12 if you have an allergy to:
- aclidinium bromide, formoterol fumarate dihydrate or any of the other ingredient of this medicine listed at the end of this leaflet.
Some of the symptoms of an allergic reaction may include:
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body
- rash, itching or hives on the skin.
Do not use this medicine after the expiry date printed on the pack or if the packaging is torn or shows signs of tampering.
In that case, return it to your pharmacist.
Before you start to use it
Tell your doctor if you have any of the following medical conditions:
- if you have asthma
- if you have had heart problems recently
- if you have epilepsy
- if you have thyroid gland problems (thyrotoxicosis)
- if you have a tumour in one of your adrenal glands (phaeochromocytoma)
- if you have difficulty passing urine
- if you see halos around lights and coloured images, have eye pain or discomfort or temporary blurring of vision (narrow angle glaucoma)
- if you have rare hereditary galactose intolerance, total lactase deficiency or glucose-galactose malabsorption.
Your doctor may want to take special precautions if you have any of the above conditions.
BRIMICA GENUAIR 340/12 may cause dryness in the mouth, which may cause tooth decay after using your medicine for a long time. Therefore, please remember to pay attention to oral hygiene.
Tell your doctor if you are pregnant, intend to become pregnant or are breastfeeding.
BRIMICA GENUAIR 340/12 is not recommended for use during pregnancy or while breastfeeding. If it is necessary for you to use this medicine during pregnancy or while breastfeeding, your doctor will discuss with you the benefits and risks involved.
Children and adolescents
BRIMICA GENUAIR 340/12 is not for use in children or adolescents below 18 years of age.
Taking other medicines
Tell your doctor or pharmacist if you are taking any other medicines, including any that you get without a prescription from your pharmacy, supermarket or health food shop.
Some medicines and BRIMICA GENUAIR 340/12 may interfere with each other. These include:
- other medicines used to treat breathing difficulties
- medicines that lower the amount of potassium in your blood. These include:
- steroids that you take by mouth (such as prednisolone)
- diuretics (such as furosemide or hydrochlorothiazide)
- certain medicines used to treat breathing conditions (such as theophylline).
- medicines called beta blockers that may be used to treat high blood pressure or other heart problems (such as atenolol or propranolol) or to treat glaucoma (such as timolol)
- medicines which can cause a change in your heart trace (electrocardiogram) known as QT interval prolongation. These include medicines for the treatment of:
- depression, such as monoamine oxidase inhibitors or tricyclic antidepressants
- bacterial infections (such as erythromycin, clarithromycin, telithromycin)
- allergic reaction (such as anti-histamines).
You may need to take different amounts of your medicines or you may need to take different medicines. Your doctor and pharmacist will advise you.
If you have not told your doctor about any of these things, tell him/ her before you start using BRIMICA GENUAIR 340/12.
How to use BRIMICA GENUAIR 340/12
Follow all directions given to you by your doctor and pharmacist carefully.
These directions may differ from the information contained in the leaflet.
If you do not understand the instructions, ask your doctor or pharmacist for help.
How much to use
The recommended dose is one inhalation twice a day in the morning and night, 12 hours apart.
The effects of BRIMICA GENUAIR 340/12 last for 12 hours; therefore, you should try to use your BRIMICA GENUAIR 340/12 inhaler at the same time every morning and at night. This ensures that there is always enough medicine in your body to help you breathe more easily throughout the day and night. It will also help you to remember to use it.
How to use
See instructions at the end of this leaflet or refer to the booklet contained within the carton for instructions on how to use the BRIMICA GENUAIR 340/12 inhaler.
If you are not sure of how to use BRIMICA GENUAIR 340/12, ask your doctor or pharmacist.
How long to use
Continue to use this medicine for as long as your doctor tells you to.
If it helps your breathing problems, your doctor may want you to keep using it for a long time.
This medicine helps to control your condition but it does not cure it.
If you want to stop treatment, first talk to your doctor, as your symptoms may worsen.
If you forget to use it
If you forget to have a dose of BRIMICA 340/12, inhale the dose as soon as you remember. However, if it is nearly time for your next dose, skip the missed dose.
Do not take a double dose to make up for the one that you missed.
This may increase the chance of you getting an unwanted side effect.
If you have trouble remembering when to use your medicine, ask your pharmacist for some hints.
If you use too much (overdose)
Immediately telephone your doctor or the Poisons Information Centre (telephone 13 11 26) for advice, or go to Accident and Emergency at the nearest hospital, if you think that you or anyone else may have used too much BRIMICA GENUAIR 340/12. Do this even if there are no signs of discomfort or poisoning.
While you are using BRIMICA GENUAIR 340/12
Things you must do
Use this medicine exactly as your doctor has prescribed. Try not to miss any doses and use it even if you feel well.
If you do not follow your doctor’s instructions, you may not get relief from your breathing problems or you may have unwanted side effects.
If you find that the usual dose of BRIMICA 340/12 is not giving as much relief as before, or does not last as long as usual, contact your doctor so that your condition can be checked.
This is important to ensure your COPD is controlled properly.
If you become pregnant while using this medicine, tell your doctor.
Your doctor can discuss with you the risks of using it while you are pregnant.
If you are about to be started on any new medicine, remind your doctor and pharmacist that you are using BRIMICA GENUAIR 340/12.
Tell any other doctors, dentists, and pharmacists who treat you that you are using this medicine.
Things you must not do
Do not exceed the recommended daily dose – it will not help you to do this.
Do not take any other medicines for your breathing problems without checking with your doctor.
Do not give this medicine to anyone else, even if their condition seems similar to yours.
Do not use it to treat any other complaints unless your doctor tells you to.
Things to be careful of
Be careful driving, operating machinery or doing jobs that require you to be alert until you know how BRIMICA GENUAIR 340/12 affects you.
This medicine may cause headache, dizziness or blurred vision. If you are affected by either of these side effects do not drive or use machinery until the headache has cleared and your vision has returned to normal.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are using BRIMICA GENUAIR 340/12.
All medicines may have some unwanted side effects. Sometimes they are serious, but most of the time they are not. You may need medical attention if you get some of the side effects.
Do not be alarmed by these lists of possible side effects. You may not experience any of them. Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor or pharmacist if you notice any of the following and they worry you:
- headache
- inflammation of the sinuses (sinusitis)
- runny nose or sneezing (rhinitis)
- common cold or flu symptoms
- cough
- increased mucous (sputum)
- diarrhoea, vomiting or nausea
- dry mouth
- changes in voice
- changes in sense of taste
- sore mouth or throat from inhaling the powder or irritation in the throat
- painful swelling and sores inside the mouth
- cellulitis
- itchy rash
- being unable to sleep
- fatigue
- dizziness
- muscle aching, cramps or spasms
- chest pain
- trembling, shakiness or agitation
- nervousness or anxiety
- infection in the tissues at the base of a tooth (tooth abscess)
- blurred vision
Tell your doctor as soon as possible if you notice any of the following:
- palpitations (feeling that your heartbeat is unusually fast or irregular)
- painful and/or frequent urination – these may be signs of a urinary tract infection
- difficulty passing urine (urinary retention)
- difficulty in breathing.
The above list includes side effects that may require medical attention.
Tell your doctor immediately or go to Accident and Emergency at your nearest hospital if any of the following happen:
- you develop signs of an allergic reaction such as swelling of the face, lips, tongue, throat, eyes or other part of the body; difficulty in breathing, palpitations, severe dizziness or fainting; redness, itching or rash on the skin
- if you get tightness of the chest, coughing, wheezing or breathlessness immediately after using the medicine. These may be signs of a condition called paradoxical bronchospasm. Your doctor may consider other treatment for your COPD.
Tell your doctor or pharmacist if you notice anything else that is making you feel unwell.
After using BRIMICA GENUAIR 340/12
Storage
Keep the BRIMICA GENUAIR 340/12 inhaler protected in the sealed pouch until you need to start using it.
The inhaler must be used within 60 days of opening the pouch.
Keep your inhaler in a cool dry place where the temperature stays below 30°C.
Do not store BRIMICA GENUAIR 340/12 or any other medicine in the bathroom or near a sink. Do not leave it on a window sill or in the car.
Heat and dampness can destroy some medicines.
Keep it where children cannot reach it.
A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop using the inhaler or the expiry date has passed, ask your pharmacist how to dispose of it properly.
Product description
What it looks like
BRIMICA 340/12 is a white or almost white powder.
The BRIMICA GENUAIR 340/12 inhaler device is white coloured with an integral dose indicator and an orange dosage button. The mouthpiece is covered with an orange removable protective cap. It is supplied in a sealed pouch.
Ingredients
Active Ingredients
Each delivered dose contains 396 micrograms aclidinium bromide (equivalent to 340 micrograms aclidinium) and 12 micrograms of formoterol fumarate dihydrate.
Inactive Ingredient
BRIMICA GENUAIR 340/12 also contains lactose monohydrate.
Supplier
BRIMICA GENUAIR 340/12 is supplied in Australia by:
A. Menarini Australia Pty Ltd
Level 8, 67 Albert Avenue
Chatswood NSW 2067
Medical Information: 1800 644 542
BRIMICA® is a registered trademark of Almirall, S.A.
GENUAIR® is a registered trademark of AstraZeneca AB
Australian Registration Number(s):
AUST R 224899
This leaflet was revised in January 2023
For the most up to date version of this document, please go to www.menarini.com.au/cmi
[vA07-0]
Instructions for Use
Getting Started
Before using the BRIMICA GENUAIR 340/12 inhaler, please read the full instructions.
You should become familiar with the parts of your inhaler (see image 1).
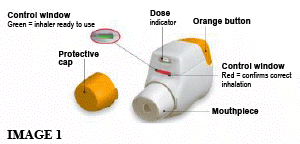
Before Use
- Before first use, tear the sealed pouch along the notch and remove the inhaler.
- Do not press the orange button until you are ready to take a dose.
- When you are about to take your dose of medicine, remove the protective cap by LIGHTLY SQUEEZING THE ARROWS marked on each side and pulling outwards (see image 2).

Step 1: Activate your inhaler
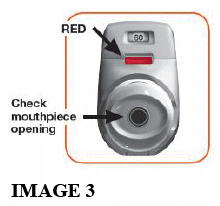
1.1 Look to see that nothing is blocking the mouthpiece (see image 3).
1.2 Look at the coloured control window and it should be red (see image 3).
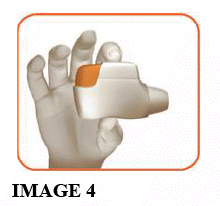
1.3 Hold the inhaler horizontally with the mouthpiece towards you and the orange button facing straight up (see image 4).
Make sure the orange button is on top. DO NOT TILT.
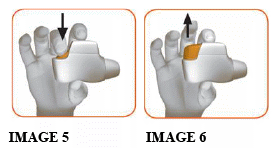
1.4 Press the orange button all the way down to load your dose (see image 5). When you press the button all the way down, the control window changes from red to green.
1.5 Release the orange button (see image 6).
Make sure you release the orange button so the inhaler can work correctly.
Stop and Check: Make sure your dose is ready for inhalation
1.6 Make sure the coloured control window has changed to GREEN (see image 7).
- The green control window confirms that your medicine is ready for inhalation.
IF THE COLOURED CONTROL WINDOW STAYS RED AFTER PRESSING THE ORANGE BUTTON ALL THE WAY DOWN, GO BACK TO 'STEP 1 ACTIVATE YOUR INHALER' AND REPEAT STEPS 1.1 TO 1.6.

Step 2: Inhale your dose
Read steps 2.1 to 2.7 fully before use. DO NOT HOLD THE ORANGE BUTTON DOWN WHILE INHALING. Do not tilt.
2.1 Before bringing the inhaler to your mouth, breathe out completely away from the mouthpiece. Do not breathe out into the mouthpiece of the inhaler.
2.2 Hold your head upright and close your lips tightly around the mouthpiece of the inhaler.
2.3 Breathe in STRONGLY and DEEPLY through your mouth (see image 8). This strong, deep breath pulls the medicine through the inhaler into your lungs.
While you breathe in you will hear a “CLICK” which signals that you are inhaling correctly. Keep breathing in for as long as possible even after you have heard the “CLICK” to be sure you get the full dose.
ATTENTION: DO NOT HOLD THE ORANGE BUTTON DOWN WHILE YOU ARE INHALING. DO NOT TILT.

2.4 Remove the inhaler from your mouth.
2.5 Hold your breath for as long as is comfortable.
2.6 Breathe out slowly through your nose, away from the mouthpiece.
Some patients may experience a grainy sensation in their mouth, or a slightly sweet or bitter taste when inhaling the medicine. Do not take an extra dose if you do not taste or feel anything after inhaling.
Stop and Check: Make sure you have inhaled correctly
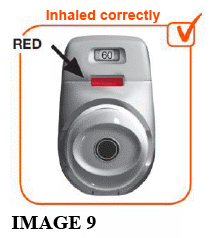
2.7 Make sure the control window is now RED (see image 9). This confirms that you have inhaled your full dose correctly.
IF THE COLOURED CONTROL WINDOW IS STILL GREEN, THIS MEANS YOU HAVE NOT INHALED YOUR FULL DOSE CORRECTLY. GO BACK TO 'STEP 2 INHALE YOUR DOSE' AND REPEAT STEPS 2.1 TO 2.7.
- If the control window still does not change to RED, you may have forgotten to release the orange button before inhaling or may not have inhaled correctly. If that happens, try again.
Make sure you have RELEASED the orange button and take a STRONG DEEP breath in through the mouthpiece.
Note: If you are unable to inhale correctly after several attempts, consult your doctor.
- Once the control window has turned red, replace the protective cap by pressing it back onto the mouthpiece (see image 10). This will prevent moisture from getting inside the mouthpiece of your inhaler.

Additional Information
What should you do if you accidentally prepared a dose?
Store your inhaler with the protective cap in place until it is time to inhale your dose, then remove the cap and start at Step 1.6.
How should you clean the inhaler?
You DO NOT NEED to clean your inhaler. However, if you wish to clean it you should do so by wiping the outside of the mouthpiece with a dry tissue or paper towel.
NEVER use water to clean the inhaler, as this may damage your medicine.
How does the dose indicator work?
- The inhaler is equipped with a dose indicator to show you approximately how many doses are left in the inhaler.
- On first use, every inhaler contains at least 60 doses, or at least 30 doses, depending on the pack size.
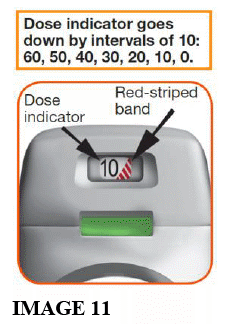
- Each time you load a dose by pressing the orange button all the way down, the dose indicator moves down slowly displaying intervals of 10 (60, 50, 40, 30, 20, 10, 0) (see image 11).
When should you get a new inhaler?
You should get a new inhaler:
- When a RED STRIPED BAND appears in the dose indicator (see image 11); this means you are nearing your last dose, or
- If your inhaler appears to be damaged or if you lose the cap, or
- If your inhaler is empty (see 'How do you know that your inhaler is empty?').
How do you know that your inhaler is empty?
- When 0 (zero) appears in the middle of the dose indicator, you should continue using any doses remaining in the inhaler.
- When the last dose has been prepared for inhalation, the orange button will not return to its full upper position, but will be locked in a middle position (see image 12). Even though the orange button is locked, your last dose may still be inhaled. After that, the inhaler cannot be used again and you should start using a new BRIMICA GENUAIR 340/12 inhaler.


