CORDILOX® SR
| Consumer Medicine Information (CMI) summary |
The full CMI on the next page has more details. If you are worried about using this medicine, speak to your doctor or pharmacist.
| 1. Why am I using CORDILOX SR? |
CORDILOX SR contains the active ingredient verapamil hydrochloride. CORDILOX SR is used for high blood pressure (hypertension) and angina (chest pain). For more information, see Section 1. Why am I using CORDILOX SR? in the full CMI.
| 2. What should I know before I use CORDILOX SR? |
Do not use if you have ever had an allergic reaction to CORDILOX SR or any of the ingredients listed at the end of the CMI.
Talk to your doctor if you have any other medical conditions, take any other medicines, or are pregnant or plan to become pregnant or are breastfeeding.
For more information, see Section 2. What should I know before I use CORDILOX SR? in the full CMI.
| 3. What if I am taking other medicines? |
Some medicines may interfere with CORDILOX SR and affect how it works. A list of these medicines is in Section 3. What if I am taking other medicines? in the full CMI.
| 4. How do I use CORDILOX SR? |
- The usual dose of CORDILOX SR is once daily or they may be taken twice daily. More instructions can be found in Section 4. How do I use CORDILOX SR? in the full CMI.
| 5. What should I know while using CORDILOX SR? |
| Things you should do |
|
| Things you should not do |
|
| Driving or using machines |
|
| Drinking alcohol |
|
| Looking after your medicine |
|
For more information, see Section 5. What should I know while using CORDILOX SR? in the full CMI.
| 6. Are there any side effects? |
Tell your doctor if you notice any of the following and they worry you: constipation, dizziness, light-headedness, feeling sick, upset stomach, headache, tiredness and flushing. These are more common side effects of your medicine.
Tell your doctor immediately if you notice any of the following: chest pain, fainting, irregular heart beat, shortness of breath which may occur with swelling of feet and legs due to fluid built up, fever, upper stomach pain, feeling generally unwell, severe blisters, skin rash, itching or flaking skin.
For more information, including what to do if you have any side effects, see Section 6. Are there any side effects? in the full CMI
CORDILOX® SR
Active ingredient: verapamil hydrochloride
| Consumer Medicine Information (CMI) |
This leaflet provides important information about using CORDILOX SR. You should also speak to your doctor or pharmacist if you would like further information or if you have any concerns or questions about using CORDILOX SR.
The letters SR in the name CORDILOX SR stand for "sustained release". This means the medicine is released into the blood over an extended period of time, usually allowing the medicine to be taken once a day.
Where to find information in this leaflet:
1. Why am I using CORDILOX SR?
2. What should I know before I use CORDILOX SR?
3. What if I am taking other medicines?
4. How do I use CORDILOX SR?
5. What should I know while using CORDILOX SR?
6. Are there any side effects?
7. Product details
| 1. Why am I using CORDILOX SR? |
CORDILOX SR contains the active ingredient verapamil hydrochloride. CORDILOX SR belongs to a group of medicines called calcium channel blockers or calcium antagonists. They work by opening up blood vessels, which lets more blood and oxygen reach the heart and at the same time lowers high blood pressure.
CORDILOX SR does not change the amount of calcium in your blood or bones. Calcium in your diet or in calcium supplements will not interfere with the way CORDILOX SR works.
CORDILOX SR is used to treat high blood pressure (hypertension) and angina (chest pain).
CORDILOX SR is not recommended for use in children under the age of 18, as there have been no studies of its effects in this age group.
There is no evidence that CORDILOX SR is addictive.
| 2. What should I know before I use CORDILOX SR? |
Warnings
Do not use CORDILOX SR if:
- you are allergic to verapamil hydrochloride, or any of the ingredients listed at the end of this leaflet. Some of the symptoms of an allergic reaction may include: shortness of breath, wheezing or difficulty breathing, swelling of the face, lips, tongue or other parts of the body, rash, itching or hives on the skin.
Always check the ingredients to make sure you can use this medicine. - You have certain heart conditions (such as heart failure, a very slow heart rate, heart conduction problems, some irregular heartbeats or disease of the heart muscle).
- You have low blood pressure, also called hypotension.
- You are taking medicines containing Ivabradine, Direct Oral Anticoagulants (DOACs) such as dabigatran (in certain situations).
- The packaging is torn or shows signs of tampering.
Check with your doctor if you have or have had any of the following medical condition:
- any other heart problems
- blood vessel (circulatory) disease or a stroke
- liver or kidney problems
- neuromuscular conditions such as Duchenne muscular dystrophy, myasthenia gravis, Lambert-Eaton syndrome.
During treatment, you may be at risk of developing certain side effects. It is important you understand these risks and how to monitor for them. See additional information under Section 6. Are there any side effects?
Pregnancy and breastfeeding
Tell your doctor if you are pregnant or plan to become pregnant.
CORDILOX SR may affect your baby if you take it in pregnancy. Your doctor will discuss the possible risks and benefits of taking CORDILOX SR during pregnancy.
Tell your doctor if you are breastfeeding or plan to breastfeed.
CORDILOX SR passes into breast milk. Your doctor will discuss the possible risks and benefits of taking CORDILOX SR when breastfeeding.
| 3. What if I am taking other medicines? |
Tell your doctor or pharmacist if you are taking any other medicines, including any medicines, vitamins or supplements that you buy without a prescription from your pharmacy, supermarket or health food shop.
Some medicines may interfere with CORDILOX SR and affect how it works.
Medicines to treat heart problems or high blood pressure:
- Beta-blockers e.g. atenolol, propranolol, metoprolol
- Ivabradine
- Digoxin
- Medicines used to control irregular heartbeat e.g. quinidine, flecainide, amiodarone, disopyramide
- Medicines used to control high blood pressure, especially prazosin or terazosin
Medicines used to lower cholesterol:
- Statins such as atorvastatin or simvastatin
Medicines used to treat or prevent blood clots (sometimes referred to as "blood thinners"):
- Direct Oral Anticoagulants (DOACs) such as dabigatran
- Aspirin
Medicines used to treat or prevent gout:
- Colchicine, sulfinpyrazone
Medicines used to treat psychological problems:
- Any medicines to treat depression, or psychosis. Such as St John's Wort, imipramine, buspirone, midazolam or lithium
Medicines to treat epilepsy or seizures:
- Phenytoin, carbamazepine, phenobarbital
Medicines to treat or prevent organ transplant rejection:
- Cyclosporin, everolimus, sirolimus, tacrolimus
Medicines used to treat infections or tuberculosis:
- Such as erythromycin, clarithromycin, telithromycin or rifampicin
Medicines used in surgical procedures:
- General anaesthetics used for inducing sleep
- Muscle relaxants
Medicines used in the treatment of Human Immunodeficiency Virus (HIV):
- Such as ritonavir
Other medicines that may react with CORDILOX SR:
- Theophylline, a medicine used to treat asthma
- Doxorubicin, a medicine used to treat certain cancers
- Cimetidine, a medicine commonly used to treat stomach ulcers and reflux
- Metformin, glibenclamide, medicines used to treat diabetes
- Aspirin
- Almotriptan
Avoid alcohol while using CORDILOX SR. You may experience greater blood pressure lowering effects than usual.
Avoid grapefruit juice, as this may increase the blood levels of verapamil.
Check with your doctor or pharmacist if you are not sure about what medicines, vitamins or supplements you are taking and if these affect CORDILOX SR.
| 4. How do I use CORDILOX SR? |
How much to take
- Your doctor will tell you how many tablets you will need to take each day and when to take them. This depends on your condition and whether you are taking any other medicines.
- The usual dose of CORDILOX SR is once daily or they may be taken twice daily.
- Treatment with CORDILOX SR is usually long term.
Keep taking CORDILOX SR for as long as your doctor recommends.
When to take CORDILOX SR
- Take CORDILOX SR with food.
How to take CORDILOX SR
- Swallow CORDILOX SR with a glass of water.
- Do not crush or chew CORDILOX SR tablets.
- CORDILOX SR tablets can be broken in half if your doctor has prescribed half a tablet.
If you forget to use CORDILOX SR
If it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to. Otherwise, take it as soon as you remember, and then go back to taking your medicine as you would normally.
Do not take a double dose to make up for the dose you missed.
This may increase the chance of you getting an unwanted side effect.
If you miss more than one dose, or are not sure what to do, check with your doctor or pharmacist.
If you use too much CORDILOX SR
If you think that you have used too much CORDILOX SR, you may need urgent medical attention.
You should immediately:
- phone the Poisons Information Centre
(Australia telephone 13 11 26) for advice, or - contact your doctor, or
- go to the Emergency Department at your nearest hospital.
You should do this even if there are no signs of discomfort or poisoning.
If you take too much CORDILOX SR, you may have difficulty breathing, a slow heartbeat, chest pain, feel very faint or collapse; however, because this medicine is released into the blood over an extended period of time, you may not notice these symptoms immediately.
| 5. What should I know while using CORDILOX SR? |
Things you should do
- If you become pregnant while taking CORDILOX SR, tell your doctor.
- Tell all doctors, dentists and pharmacists who are treating you that you are taking CORDILOX SR.
- If you are going to have surgery including dental surgery, tell your doctor or dentist that you are taking CORDILOX SR.
- Visit your doctor regularly so that they can check on your progress.
- Your doctor may ask you to have blood tests to check your liver from time to time.
Call your doctor straight away if you:
- are being treated for angina, tell your doctor if you continue to have angina attacks or if they become more frequent while you are using CORDILOX SR.
Remind any doctor, dentist or pharmacist you visit that you are using CORDILOX SR.
Things you should not do
- Do not stop taking CORDILOX SR or lower the dosage without checking with your doctor.
- Do not take CORDILOX SR with grapefruit or its juice.
- Do not give CORDILOX SR to anyone else, even if they have the same condition as you.
- Do not take CORDILOX SR to treat any other complaints unless your doctor tells you to.
Be careful getting up from a sitting position
- Dizziness, light-headedness or fainting may occur, especially when you get up quickly. Getting up slowly may help.
Driving or using machines
Be careful before you drive or use any machines or tools until you know how CORDILOX SR affects you.
CORDILOX SR may cause dizziness, light-headedness or tiredness in some people. If this occurs, do not drive, operate machinery or do anything else that could be dangerous.
Drinking alcohol
Tell your doctor if you drink alcohol.
If you drink alcohol while taking CORDILOX SR, dizziness or light-headedness may be worse.
Looking after your medicine
- Keep CORDILOX SR below 25°C.
- Keep your tablets in the pack until it is time to take them.
Follow the instructions in the carton on how to take care of your medicine properly.
Store it in a cool dry place away from moisture, heat or sunlight; for example, do not store it:
- in the bathroom or near a sink, or
- in the car or on window sills.
Keep it where young children cannot reach it.
Getting rid of any unwanted medicine
If you no longer need to use this medicine or it is out of date, take it to any pharmacy for safe disposal.
Do not use this medicine after the expiry date.
| 6. Are there any side effects? |
All medicines can have side effects. If you do experience any side effects, most of them are minor and temporary. However, some side effects may need medical attention.
As with most medicines, if you are over 65 years of age you may have an increased chance of getting side effects. Report any side effects to your doctor promptly.
See the information below and, if you need to, ask your doctor or pharmacist if you have any further questions about side effects.
Less serious side effects
| Less serious side effects | What to do |
| Speak to your doctor if you have any of these more common side effects and they worry you. |
Serious side effects
| Serious side effects | What to do |
| Call your doctor straight away, or go straight to the Emergency Department at your nearest hospital if you notice any of these serious side effects. |
Tell your doctor or pharmacist if you notice anything else that may be making you feel unwell.
Other side effects not listed here may occur in some people.
Reporting side effects
After you have received medical advice for any side effects you experience, you can report side effects to the Therapeutic Goods Administration online at www.tga.gov.au/reporting-problems. By reporting side effects, you can help provide more information on the safety of this medicine.
Always make sure you speak to your doctor or pharmacist before you decide to stop taking any of your medicines.
| 7. Product details |
This medicine is only available with a doctor's prescription.
What CORDILOX SR 180mg contains
| Active ingredient (main ingredient) | verapamil hydrochloride |
| Other ingredients (inactive ingredients) | sodium alginate microcrystalline cellulose povidone magnesium stearate hypromellose macrogol 400 macrogol 6000 purified talc titanium dioxide iron oxide red glycol/butylene glycol montanate |
| Potential allergens | sulfites |
What CORDILOX SR 240mg contains
| Active ingredient (main ingredient) | verapamil hydrochloride |
| Other ingredients (inactive ingredients) | sodium alginate powdered cellulose povidone magnesium stearate hypromellose purified talc macrogol 400 macrogol 6000 titanium dioxide quinoline yellow indigo carmine glycol/butylene glycol montanate purified water |
| Potential allergens | sulfites |
Do not take this medicine if you are allergic to any of these ingredients.
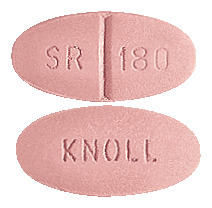
What CORDILOX SR looks like
CORDILOX SR 180mg is old rose, oval film coated tablet. ‘KNOLL’ on one face and ‘SR’; score; ‘180’ on the other face (AUST R 54033).
CORDILOX SR 240mg is light green, oblong film-coated tablet (curved tooling). Score and 2 logos on one face and score on the other face (AUST R 10681).
Who distributes CORDILOX SR
Viatris Pty Ltd
Level 1, 30 The Bond
30-34 Hickson Road
Millers Point NSW 2000
www.viatris.com.au
Phone: 1800 274 276
This leaflet was prepared in December 2022.
CORDILOX® SR is a Viatris company trade mark
CORDILOX SR_cmi\Dec22/00
Published by MIMS February 2023