What is in this leaflet
This leaflet answers some common questions about Cyproterone Sandoz.
It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking this medicine against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine. You may need to read it again.
What Cyproterone Sandoz is used for
Cyproterone Sandoz is an anti-androgen medicine.
Androgens such as testosterone are natural male sex hormones which are also produced, to a slight extent, in females.
MEN:
In men, androgens may help cancer cells to grow in some types of prostate cancer. By blocking these hormones, Cyproterone Sandoz may slow or stop the growth of cancer. It can also be used in combination with other medicines or following surgical removal of the testes to treat side effects such as “hot flushes” or “sweats” and to prevent any initial worsening of the disease.
Cyproterone Sandoz is also used to reduce abnormal sex drive in men.
WOMEN:
In women, androgens may increase hair growth, loss of scalp hair and secretion of oil from the sweat glands. By blocking these hormones, Cyproterone Sandoz may slow or stop excessive hairiness, loss of scalp hair, acne, oily skin and dandruff.
Ask your doctor if you have any questions about why this medicine has been prescribed for you. Your doctor may have prescribed it for another reason.
This medicine is available only with a doctor’s prescription.
Before you take Cyproterone Sandoz
When you must not take it
Do not take this medicine if you have an allergy to:
- cyproterone acetate, the active ingredient, or to any of the other ingredients listed at the end of this leaflet under Product Description.
Some of the symptoms of an allergic reaction may include:
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body
- rash, itching or hives on the skin.
Do not give this medicine to children. This medicine should not be taken by children and adolescents below 18 years of age or girls who have not completed puberty.
Do not take this medicine if you are pregnant or suspect you may be pregnant. It may affect your developing baby if you take it during pregnancy.
Do not breastfeed if you are taking this medicine. The active ingredient in Cyproterone Sandoz passes into breast milk and there is a possibility that your baby may be affected.
Do not take this medicine if you have any of the following medical conditions:
- liver disease, previous or existing liver tumours unless they are caused by metastases from prostate cancer (your doctor would have told you if you have this)
- Dubin-Johnson or Rotor syndrome (your doctor would have told you if you have either of these conditions)
- history of jaundice (yellow skin or eyes), herpes, or persistent itching during a previous pregnancy
- previous or existing benign brain tumour (meningioma).
- wasting disease (a disease causing muscle loss or loss of strength, with the exception of prostate cancer)
- severe and persistent depression
- previous or existing conditions relating to formation of blood clots
- severe diabetes with blood vessel changes (your doctor would have told you if you have this)
- sickle-cell anaemia (your doctor would have told you if you have this)
Cyproterone Sandoz contains lactose monohydrate. If you have been told by your doctor that you have an intolerance to some sugars, contact your doctor before taking Cyproterone Sandoz.
Do not take this medicine after the expiry date printed on the pack or blister. The expiry date is printed on the carton and on each blister after “EXP” (e.g. 11 18 refers to November 2018). The expiry date refers to the last day of that month. If it has expired return it to your pharmacist for disposal.
Do not take this medicine if the packaging is torn or shows signs of tampering. If the packaging is damaged, return it to your pharmacist for disposal.
If you are not sure whether you should start taking this medicine, talk to your doctor.
Before you start to take it
Tell your doctor if you have allergies to any other medicines, foods, preservatives or dyes.
Tell your doctor if you have or have had any of the following medical conditions, especially the following:
- diabetes
- history of blood clotting or sickle cell anaemia
- osteoporosis, a family history of osteoporosis or risk factors for developing osteoporosis (such as smoking, a diet low in calcium, poor mobility, a slight build or treatment with steroid medicines)
Tell your doctor if you are pregnant or plan to become pregnant or are breastfeeding. If taken during pregnancy, Cyproterone Sandoz may lead to signs of feminisation in the male foetus. Therefore, your doctor will check that you are not pregnant before you start taking Cyproterone Sandoz. Women should use a reliable form of contraception while taking Cyproterone Sandoz.
Tell your doctor if fertility after treatment is important. For men it is recommended that a sperm count is taken to establish fertility before commending Cyproterone Sandoz. It can take 3-20 months for fertile sperm production to be re-established after stopping this medicine.
The long-term effects of Cyproterone Sandoz on female fertility are not known.
If you have not told your doctor about any of the above, tell him/her before you start taking Cyproterone Sandoz.
Taking other medicines
Tell your doctor or pharmacist if you are taking any other medicines, including any that you get without a prescription from your pharmacy, supermarket or health food shop.
Some medicines and Cyproterone Sandoz may interfere with each other. These include:
- phenytoin, a medicine used to treat epilepsy
- medicines used to treat fungal infections, such as ketoconazole, itraconazole, clotrimazole
- rifampicin, an antibiotic used to treat infections such as tuberculosis and leprosy
- ritonavir, a medicine used in the treatment of HIV
- St John’s Wort, a herbal remedy used to treat mood disorders
- statins (HMGCoA inhibitors), medicines used to lower cholesterol levels in people with or at risk of cardiovascular disease
- medicines used to treat diabetes
These medicines may be affected by Cyproterone Sandoz or may affect how well it works. You may need different amounts of your medicines, or you may need to take different medicines.
Your doctor and pharmacist have more information on medicines to be careful with or avoid while taking this medicine.
How to take Cyproterone Sandoz
Follow all directions given to you by your doctor or pharmacist carefully. They may differ from the information contained in this leaflet.
If you do not understand the instructions, ask your doctor or pharmacist for help.
How much to take
Your doctor will advise of the most suitable dose for you to take.
Do not alter the dose yourself. Your doctor will advise you if changing the dose is necessary.
MEN:
Prostate cancer:
The usual daily dose is 50-300 mg (half to three tablets) of Cyproterone Sandoz 100 mg.
Your doctor may request that you take Cyproterone Sandoz with other medicines and/or change your dose during treatment.
Reduction of abnormal sex drive:
Generally, treatment is started with one tablet of Cyproterone Sandoz 50 mg twice daily and may be increased to 100 mg twice daily or three times daily before reducing gradually to the lowest effective dose. Your doctor may change your dose during treatment.
WOMEN:
If you are of childbearing age, you should commence your tablet taking on the 1st day of your cycle (= 1st day of bleeding). If you have no menstrual periods (amenorrhea) your treatment can start immediately. In this case, the first day of treatment is to be regarded as the 1st day of the cycle.
Starting from day 1 take 50-100 mg (as advised by your doctor) of Cyproterone Sandoz daily from 1st to the 10th day of the cycle (=for 10 days). Additionally, your doctor will advise the most appropriate contraceptive for you to take to provide the necessary contraceptive protection and to stabilise your cycle.
Your doctor will re-evaluate your treatment when you reach menopause. Long-term use (years) of Cyproterone Sandoz should be avoided.
If you are postmenopausal or have had a hysterectomy, Cyproterone Sandoz may be administered alone. The usual dose is 25-50mg of Cyproterone Sandoz once daily for 21 days, followed by a 7-day-tablet-free interval.
Shortness of breath may occur at high doses.
How to take it
Swallow the tablets whole with some liquid after meals.
When to take it
Take your medicine after meals at about the same time each day. Taking it at the same time each day will have the best effect. It will also help you remember when to take it.
Missed Cyproterone Sandoz tablets may diminish the effectiveness of treatment and may lead to breakthrough bleeding in women.
How long to take it
Continue taking your medicine for as long as your doctor tells you.
This medicine helps to control your condition, but does not cure it. It is important to keep taking your medicine even if you feel well.
If you forget to take it
If it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to.
Otherwise, take it as soon as you remember, and then go back to taking your medicine as you would normally.
Do not take a double dose to make up for the dose that you missed. This may increase the chance of you getting an unwanted side effect.
If you are also taking an oral contraceptive and more than 12 hours has elapsed from the time Cyproterone Sandoz was due to be taken, note that contraceptive protection may be reduced and thus there is an increased risk of becoming pregnant.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering to take your medicine, ask your pharmacist for some hints.
If you take too much (overdose)
Immediately telephone your doctor or the Poisons Information Centre (telephone 13 11 26) for advice, or go to Accident and Emergency at the nearest hospital, if you think that you or anyone else may have taken too much Cyproterone Sandoz. Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
While you are taking Cyproterone Sandoz
Things you must do
If you are about to be started on any new medicine, remind your doctor and pharmacist that you are taking Cyproterone Sandoz.
Tell any other doctors, dentists, and pharmacists who treat you that you are taking this medicine.
For females taking the “Pill” during treatment:
If light bleeding or spotting occurs during the three weeks in which the tablets are being taken, do not stop taking your tablets.
However, if unusual bleeding patterns continue, tell your doctor.
If you are a female taking an oral contraceptive during treatment, tell your doctor if your period does not occur during the tablet-free/placebo interval. Your doctor may need to check whether you are pregnant before you can continue treatment.
For males taking Cyproterone Sandoz to reduce abnormal sex drive:
You should consider undertaking additional measures such as therapy or counselling in order to take advantage of the period of reduced drive. These measures may assist in achieving personal and social reorientation.
Keep all of your doctor’s appointments so that your progress can be checked. Your doctor may do some tests (such as liver or blood tests) from time to time to make sure the medicine is working and to prevent unwanted side effects.
Your doctor will check your liver function during treatment Cyproterone Sandoz and whenever any symptoms or signs suggesting liver problems are observed.
If you have diabetes, your doctor will monitor you to ensure that you receive the appropriate dose of oral antidiabetic or insulin whilst taking Cyproterone Sandoz.
Your doctor will also check your red-blood cell count to ensure you do not become anaemic during treatment with Cyproterone Sandoz.
Things you must not do
Do not take Cyproterone Sandoz to treat any other complaints unless your doctor tells you to.
Do not give your medicine to anyone else, even if they have the same condition as you.
Do not stop taking your medicine or lower the dosage without checking with your doctor. If you stop taking it suddenly, your condition may worsen or you may have unwanted side effects.
Things to be careful of
Be careful driving or operating machinery until you know how Cyproterone Sandoz affects you. This medicine may cause tiredness and can impair the ability to concentrate. If you have any of these symptoms, do not drive, operate machinery or do anything else that could be dangerous.
Be careful when drinking alcohol while you are taking this medicine. If you drink alcohol, tiredness and the ability to concentrate may be worse. Alcohol may prevent Cyproterone Sandoz from working as well as it should in reducing abnormal sex drive.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking Cyproterone Sandoz.
All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical attention if you get some of the side effects.
Do not be alarmed by the following lists of side effects. You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor or pharmacist if you notice any of the following and they worry you:
- tiredness, fatigue
- weight changes (increase or decrease)
- decreased sexual drive
- headache
- depressed mood
- nausea and other gastrointestinal complaints
- breast pain, changes in breast size, breast swelling and/or tenderness
- breast enlargement in men
- menstrual cycle irregularity, spotting
- hot flushes, sweating
- shortness of breath
- osteoporosis.
If you were fertile before treatment, Cyproterone Sandoz will normally prevent sperm production in men and ovulation in women. In men, fertility is usually regained within a few months of discontinuing therapy. The long term effects on female fertility are not known.
In men, Cyproterone Sandoz will also normally result in the inability to get or maintain an erection (impotence). This is usually regained within a few months of discontinuing therapy.
The above list includes the more common side effects of the medicine.
Use of cyproterone acetate has been linked to the development of meningioma (generally benign tumour). The risk increases when used for several years, or with high doses (25 mg per day and above).
Tell your doctor immediately if you notice any of the following:
- changes in vision (e.g. seeing double or blurriness),
- hearing loss or ringing in the ears,
- loss of smell,
- headaches that worsen with time,
- memory loss,
- seizures,
- weakness in your arms or legs
Tell your doctor immediately or go to Accident and Emergency at your nearest hospital if you notice any of the following:
- yellowing of the skin and/or eyes, light coloured bowel motions, dark coloured urine
- severe upper abdominal pain
- vomiting blood or material that looks like coffee grounds, bleeding from the back passage, black sticky bowel motions (stools) or bloody diarrhoea.
- sudden severe headache, loss of vision, loss of co-ordination, slurred speech, numbness, heat or swelling in the arms and legs
The above list includes serious side effects that may require medical attention. Serious side effects are rare.
Tell your doctor or pharmacist if you notice anything else that is making you feel unwell. Other side effects not listed above may also occur in some people.
After taking Cyproterone Sandoz
Storage
Keep your medicine in the original container.
If you take it out of its original container it may not keep well.
Keep your medicine in a cool dry place where the temperature stays below 30°C.
Do not store Cyproterone Sandoz or any other medicine in the bathroom or near a sink. Do not leave it on a window sill or in the car.
Heat and dampness can destroy some medicines.
Keep it where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking this medicine or the expiry date has passed, ask your pharmacist what to do with any medicine that is left over.
Return any unused medicine to your pharmacist.
Product description
What it looks like
Cyproterone Sandoz comes in two types of tablets:
Cyproterone Sandoz 50 mg – white to off white, round tablets engraved with ‘50’ over a break line on one face, plain on the other face.
Available in bottles of 20 or 50 tablets.
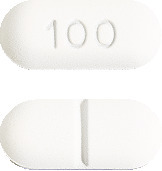
Cyproterone Sandoz 100 mg – white to off white, capsule shaped tablets with ‘100’ engraved on one face and a break line on the other face.
Available in blisters of 50 tablets.
Ingredients
Active ingredients:
- Cyproterone Sandoz 50 mg – 50 mg cyproterone acetate
- Cyproterone Sandoz 100 mg – 100 mg cyproterone acetate
Inactive ingredients:
- lactose monohydrate
- microcrystalline cellulose
- croscarmellose sodium
- povidone
- magnesium stearate.
This medicine does not contain sucrose, gluten, tartrazine or any other azo dyes.
Supplier
Cyproterone Sandoz is supplied in Australia by:
Sandoz Pty Ltd
ABN 60 075 449 553
54 Waterloo Road
Macquarie Park, NSW 2113
Australia
Tel: 1800 726 369
This leaflet was revised in March 2021.
Australian Register Number(s)
50 mg tablets: AUST R 107330 (bottles)
100 mg tablets: AUST R 107331 (blisters)
Published by MIMS May 2021

 Chemical name: 6-chloro-17αhydroxy-1α,2α-methylene-pregna- 4,6-diene-3,20-dione acetate.
Chemical name: 6-chloro-17αhydroxy-1α,2α-methylene-pregna- 4,6-diene-3,20-dione acetate.