What is in this leaflet
This leaflet answers some common questions about Diacol®.
It does not contain all the information that is known about Diacol®. It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor or pharmacist has weighed the risks of you taking Diacol® against the benefits it is expected to have for you.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine. You may need to read it again.
What Diacol® is used for
Diacol® belongs to a group of medicines that are described as purgatives (very strong laxatives). Purgatives are used to clean the bowel. They work by causing large amounts of water to be drawn into the bowel, which results in diarrhoea.
Diacol® is used in patients (adults 18 years or older) who need to have their bowel examined (by procedures such as colonoscopy). The bowel needs to be clean before your doctor can examine it properly.
Your doctor may have prescribed Diacol® for another reason. Ask your doctor if you have any questions about why Diacol® has been prescribed for you.
However, laxatives and purgatives (like Diacol®) as a group have the potential for abuse by persons with eating disorders who ‘binge’ and ‘purge’.
Before you take Diacol®
When you must not take it
Do not take Diacol® if you have an allergy to:
- Any of the ingredients listed at the end of this leaflet
- Any similar medicines to Diacol®
Symptoms of an allergic reaction may include:
- Shortness of breath
- Wheezing or difficulty breathing
- Swelling of the face, lips, tongue or other parts of the body
- Skin rash, itching or hives
Do not take Diacol® if you have, or have had, any of the following medical conditions:
- Heart disease
- Chest pain
- Perforated bowel
- Bowel problems such as blockages or constipation
- An inflamed colon (colitis) or an enlarged colon
- Hypomotility syndrome (infrequent passing of stools)
- A condition called ascites which results in a fluid build-up around the stomach which causes swelling
- Kidney Disease
- Dehydration
- Low sodium, calcium or potassium levels in the blood
- High sodium or phosphate levels in the blood
- Gastric bypass or stapling surgery
- Imperforate anus
- Paralytic ileus
- Hirschprung's disease / congenital megacolon
- Active inflammatory bowel disease
Caution should be exercised in ‘at risk’ patients such as the elderly who are more at risk of dehydration as electrolyte depletion may occur.
Diacol® is not recommended for use in children (under the age of 18), as the safety and effectiveness of Diacol® in this age group have not been established.
Do not take Diacol® after the expiry date printed on the pack or if the packaging is torn or shows signs of tampering. If you take this medicine after the expiry date has passed, it may not work as well.
If it has expired or is damaged, return it to your pharmacist for disposal.
If you are not sure when you should start taking Diacol®, talk to your doctor or pharmacist.
Before you start to take it
Tell your doctor or pharmacist if you have allergies to:
- Any other medicines
- Any other substances, such as foods, preservatives or dyes
Tell your doctor if you have or have had any medical conditions, especially the following:
- Chronic inflammatory bowel disease
- Liver disease
- Recent cardiac surgery, cardiac arrest or heart problems
- Thyroid problems
- Seizures
- Withdrawal from alcohol or benzodiazepines (medicines used to treat anxiety)
- Diabetes
Also tell your doctor if you are on a low sodium diet. Diacol® contains a large amount of sodium and the product may be harmful to you.
Tell your doctor or pharmacist if you are pregnant, intend to become pregnant, are breastfeeding or intend to breastfeed. Diacol® is not generally recommended for use in pregnant women unless the benefits of treatment outweigh the risk to the unborn baby. Your doctor will discuss the possible risks and benefits of using Diacol® during pregnancy and breastfeeding.
If you are elderly, tell your doctor your age. Your doctor may need to check if it is safe for you to take Diacol®.
Tell your doctor if you are diabetic. The liquid diet recommended with this medication may affect your blood glucose levels and adjustment of your diabetic medication may be required.
If you have not told your doctor or pharmacist about any of the above, tell them before you start taking Diacol®.
Taking other medicines
Tell your doctor or pharmacist if you are taking any other medicines, including any that you buy without a prescription from your pharmacy, supermarket or health food shop.
These include:
- Calcium channel blockers (for angina and heart conditions)
- Diuretics (water tablets)
- Lithium (for mental illness)
- Other medicines that may affect electrolyte balance
- Other bowel cleansing preparations.
- Non-steroidal anti-inflammatory drugs (for pain relief, swelling and other symptoms of inflammation, including arthritis)
- ACE Inhibitors (for high blood pressure and some other heart conditions)
- Angiotensin receptor blockers
These medicines may be affected by Diacol®, or may affect how well it works. You may need different amounts of your medicine, or you may need to take different medicines. Your doctor or pharmacist will advise you.
Medications that are taken just before or during the course of Diacol® may not be absorbed. This is due to the increased movement in the digestive tract and the watery diarrhoea that is caused by Diacol®.
These include:
- Oral contraceptives (“the pill”)
- Antibiotics
- Medicines for diabetes
Your doctor and pharmacist may have more information on medicines to be careful with or avoid while taking Diacol®.
How to take Diacol®
Follow all directions given to you by your doctor or pharmacist carefully. They may differ from the information contained in this leaflet.
If you do not understand the instructions on the bottle or in this leaflet, ask your doctor or pharmacist for help.
How much to take
The usual adult dosage of Diacol® taken for colon cleansing is 32 tablets to be taken as 2 lots, one of 20 tablets and one of 12 tablets.
How to take it
Because of the way Diacol® works, you must not eat any food at least 12 hours before you take the first dose of Diacol® and while you are taking the tablets. You should only drink clear liquids for 12 hours before taking Diacol® and while you are taking the tablets.
Clear liquids include strained fruit juice without pulp (apple, white grape, orange), water, clear broth, coffee or tea (without milk or non-dairy creamer) and all of the following that are not coloured red or purple: non-carbonated soft drinks, fruit flavoured cordials, clear ice blocks.
When to take it
In the evening on the day before the procedure, 4 tablets should be taken with at least one full glass of water (250mL) every 15 minutes for a total of 20 tablets. DO NOT TAKE MORE THAN 20 TABLETS.
On the day of the procedure (starting 3 – 5 hours before the procedure), 4 tablets should be taken with at least a full glass of water (250mL) every 15 minutes for a total of 12 tablets. DO NOT TAKE MORE THAN 12 TABLETS.
REMEMBER you need to be close to toilet facilities whilst you are taking Diacol®. Bowel movements may continue for several hours after the last dose of Diacol® has been taken.
Do NOT take additional laxatives or purgatives, particularly those containing sodium or phosphate.
You may see undigested or partially digested tablets in the watery diarrhoea stools.
How long to take it
Take Diacol® as prescribed by your doctor on the evening before and on the morning of the procedure. Do not take more than a total of 32 tablets.
If you forget to take it
Do not take a double dose to make up for forgotten individual doses. Take your next dose of Diacol® as soon as you remember and continue taking the tablets every 15 minutes until you have completed the course.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering to take your medicine, ask your pharmacist for some hints.
If you take too much (overdose)
Immediately telephone your doctor or the Poisons Information Centre (Tel 131 126), or go to the Emergency Department at your nearest hospital, if you think that you or anyone else may have taken too much Diacol®. Do this even if there are no signs of discomfort or poisoning.
You may need urgent medical attention.
If you take too much Diacol®, you may feel dehydrated, light-headed or dizzy.
While you are taking Diacol®
Things you must do
Diacol® can lead to serious dehydration and electrolyte disturbances. You must ensure that you drink the recommended amount of liquid to replace the large amounts of fluid that may be lost during bowel emptying. Ask your doctor or pharmacist to answer any questions you may have.
Things you must not do
Do not repeat the course of Diacol® for at least seven days. No other laxative, enema or other medication containing sodium phosphate should be taken.
Do not give Diacol® to anyone else, even if they have the same condition as you.
Do not take Diacol® to treat any other complaints unless your doctor or pharmacist tells you to.
Do not stop taking Diacol®, or lower the dosage, without checking with your doctor or pharmacist.
Things to be careful of
Do not attempt to drive or operate machinery during the treatment with Diacol®. It will be impractical for you to drive or operate machinery since you will have to stay close to a toilet until the purgative effect is complete. Diacol® may also make you feel light headed or dizzy or drowsy, which would make driving or operating machinery dangerous if you are affected.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking Diacol®. Diacol® helps most people that require bowel cleansing, but it may have unwanted side effects in a few people. All medicines can have side effects. Sometimes they are serious, most of the time they are not.
If you are over 65 years of age you may have an increased chance of getting side effects.
Ask your doctor or pharmacist to answer any questions you may have.
Diacol® may cause a number of diarrhoea like bowel movements. This is normal and is a result of its bowel-cleansing action. You should not be alarmed by this, but should continue to drink the recommended amount of clear fluids to prevent dehydration.
The following is a list of possible side effects. Tell your doctor or pharmacist if you notice any of the following and they worry you:
- Nausea (feeling sick)
- Vomiting
- Stomach pain
- Stomach bloating
- Dizziness
- Headache
- Light headed
- Drowsiness
- Signs of dehydration such as feeling thirsty and urinating less often
Do not be alarmed by this list. You may not experience any of them.
If the effects are severe, you may need medical treatment.
However, these side effects usually disappear within several hours of finishing Diacol® treatment.
If you get any side effects, do not stop taking Diacol® without first talking to your doctor or pharmacist.
Other side effects not listed above may also occur in some patients. Tell your doctor or pharmacist if you notice anything that is making you feel unwell.
After using Diacol®
Storage
Keep your tablets in the bottle until it is time to take them. If you take the tablets out of the bottle they will not keep well.
Keep your tablets in a cool dry place where the temperature stays below 25°C. Keep the container tightly closed.
Do not store Diacol® or any other medicine in the bathroom or near a sink.
Do not leave it on a window sill or in the car on hot days. Heat and dampness can destroy some medicines.
Keep it where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor or pharmacist tells you to stop taking Diacol® or the tablets have passed their expiry date, ask your pharmacist what to do with any that are left over.
Product description
What it looks like
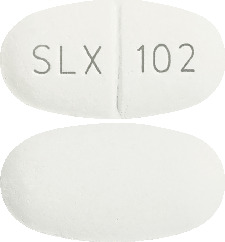
Diacol® Tablets are oval, white to off white, “SLX” bisect “102” on one side and plain on the other side.
Diacol® is available in bottles of 32 tablets. Each bottle contains 2 silica desiccant packets which are not to be consumed.
Ingredients
Active ingredients:
- Monobasic sodium phosphate (1102mg per tablet)
- Dibasic sodium phosphate (398mg per tablet)
Other ingredients:
- Magnesium stearate
- Polyethylene glycol 8000
Diacol® does not contain lactose, sucrose, gluten, tartrazine or any azo dyes.
Supplier
Diacol® is supplied by:
Fresenius Kabi Australia Pty Limited
Level 2, 2 Woodland Way, Mount Kuring-gai NSW 2080, Australia
Telephone: (61-2) 9391 5555
This leaflet was prepared on 15 April 2016.
Australian Registration Number:
AUST R 149914
Diacol® is a trademark of Fresenius Kabi Australia Pty Limited
Published by MIMS September 2019



 The serum phosphorus level remained above baseline for a median of 24 hours after the initial dose of sodium phosphate tablets (range 16 to 48 hours), with a maximum mean decrease of 18 hours in serum calcium of 0.3 mg/dL ± 0.03 mg/dL.
The serum phosphorus level remained above baseline for a median of 24 hours after the initial dose of sodium phosphate tablets (range 16 to 48 hours), with a maximum mean decrease of 18 hours in serum calcium of 0.3 mg/dL ± 0.03 mg/dL.