Dukoral®
| Consumer Medicine Information (CMI) summary |
The full CMI below has more details. If you are worried about using this medicine, speak to your doctor or pharmacist.
| 1. Why am I taking Dukoral®? |
Dukoral® contains the active ingredients Vibrio cholerae (cholera) bacteria (inactivated) and cholera toxin B subunit (recombinant). Dukoral® is a vaccine used to help prevent cholera.
For more information, see Section 1. Why am I taking Dukoral®? in the full CMI.
| 2. What should I know before I take Dukoral®? |
Do not use if you or your child have ever had an allergic reaction to Dukoral® or formaldehyde or any of the ingredients listed at the end of the CMI.
Talk to your doctor if you or your child have any other medical conditions, take any other medicines, or are pregnant or plan to become pregnant or are breastfeeding.
For more information, see Section 2. What should I know before I take Dukoral®? in the full CMI.
| 3. What if I am taking other medicines? |
Some medicines may interfere with Dukoral® and affect how it works.
A list of these medicines is in Section 3. What if I am taking other medicines? in the full CMI.
| 4. How do I take Dukoral®? |
- Follow all directions given to you by your doctor or pharmacist carefully.
- Avoid food and drink for 1 hour before and 1 hour after drinking the vaccine. Food and drink taken during this time may inactivate the vaccine.
More instructions can be found in Section 4. How do I take Dukoral®? in the full CMI.
| 5. What should I know while taking Dukoral®? |
| Things you or your child should do |
|
| Things you or your child should not do |
|
| Looking after your medicine |
|
For more information, see Section 5. What should I know while taking Dukoral®? in the full CMI.
| 6. Are there any side effects? |
The most common side effects include, diarrhoea, loose stools, stomach discomfort, vomiting, nausea, headache, cough and runny or blocked nose.
Serious side effects include, severe diarrhoea, fainting, shortness of breath and allergic reactions.
For more information, including what to do if you have any side effects, see Section 6. Are there any side effects? in the full CMI.
Dukoral®
Active ingredient(s): Vibrio cholerae (cholera) bacteria (inactivated), Recombinant cholera toxin B subunit.
| Consumer Medicine Information (CMI) |
This leaflet provides important information about using Dukoral®. You should also speak to your doctor or pharmacist if you would like further information or if you have any concerns or questions about using Dukoral®.
Where to find information in this leaflet:
1. Why am I taking Dukoral®?
2. What should I know before I take Dukoral®?
3. What if I am taking other medicines?
4. How do I take Dukoral®?
5. What should I know while taking Dukoral®?
6. Are there any side effects?
7. Product details
| 1. Why am I taking Dukoral®? |
Dukoral® contains the active ingredient Vibrio cholerae (cholera) bacteria (inactivated) and recombinant cholera toxin B subunit. Dukoral® is a vaccine used to help prevent cholera.
Dukoral® is used to protect adults and children from two years of age who are traveling to an area where there is a risk of cholera.
Dukoral® works by causing your body to produce its own protection against cholera. It does this by making substances called antibodies in the intestine wall, which fight the cholera bacteria (Vibrio cholerae) and the cholera toxin. Cholera toxin causes diarrhoea and is produced by the cholera bacteria. If a vaccinated person comes into contact with cholera bacteria or cholera toxin, the body is usually ready to destroy it.
Your body usually takes two weeks after vaccination to develop protection against diarrhoea due to cholera.
Most people will produce enough antibodies to protect against diarrhoea due to cholera. However, as with all vaccines, 100% protection cannot be guaranteed. About 85% of people can expect to be protected against cholera in the 6 months following initial vaccination. This decreases to 52% at the end of the second year, when a booster should be given if ongoing protection is needed. If more than 2 years have passed, the primary course should be repeated.
The vaccine will not give you or your child cholera.
The chance of a severe reaction from Dukoral® is very small, but the risks from not being vaccinated against cholera may be very serious.
| 2. What should I know before I take Dukoral®? |
Warnings
Do not take Dukoral® if:
- you are allergic to Dukoral®, formaldehyde, or any of the ingredients listed at the end of this leaflet.
- you have a high temperature.
- you have symptoms such as vomiting, nausea, cramps or diarrhoea.
Always check the ingredients to make sure you can use this medicine.
Talk to your doctor or pharmacist if you are not sure whether you or your child should take Dukoral®.
Check with your doctor if you or your child:
- have any other medical conditions, especially the following
- a disease that weakens your immune system (e.g. HIV/AIDS)
- low/weak immunity due to treatment with medicines such as corticosteroids, cyclosporine or other medicines used to treat autoimmune conditions or cancer (including radiation therapy)
The vaccine may provide you with a lower level of protection than it does for people with healthy immune systems. - take any medicines for any other condition
- have an infection or have been unwell. Your doctor may decide to delay vaccination until the illness has passed. A mild illness, such as a cold, is not usually a reason to delay vaccination.
- are on a controlled sodium (low salt) diet. Dukoral® contains approximately 1,200 mg sodium per dose.
- have allergies to any other medicines
- have allergies to any other substances, such as foods, preservatives or dyes.
During treatment, you may be at risk of developing certain side effects. It is important you understand these risks and how to monitor for them. See additional information under Section 6. Are there any side effects?
Pregnancy and breastfeeding
Check with your doctor if you are pregnant or intend to become pregnant. Your doctor will discuss the possible risks and benefits of having Dukoral® during pregnancy.
Talk to your doctor if you are breastfeeding or intend to breastfeed. Your doctor will discuss the possible risks and benefits of having Dukoral® while you are breastfeeding.
Children
Do not give Dukoral® to a child under 2 years of age.
There is not enough information to recommend the use of Dukoral® in children under 2 years of age.
| 3. What if I am taking other medicines? |
Tell your doctor or pharmacist if you or your child are taking any other medicines, including any medicines, vitamins or supplements that you buy without a prescription from your pharmacy, supermarket or health food shop.
Some medicines may interfere with Dukoral® and affect how it works.
Do not take any other oral medicines at the same time as Dukoral®.
Other oral medicines should not be taken for at least 1 hour before and 1 hour after you take Dukoral®.
Having other vaccines
Your doctor will tell you if you can have Dukoral® at the same time as another vaccine.
If you have to take Dukoral® and an oral typhoid vaccine, you should have the vaccines at least 8 hours apart.
Check with your doctor or pharmacist if you are not sure about what medicines, vitamins or supplements you are taking and if these affect Dukoral®.
| 4. How do I take Dukoral®? |
How much to take
- Follow the instructions provided to you by your doctor or pharmacist carefully. Your doctor or pharmacist will tell you how to take this vaccine.
- Primary Course:
Adults and children over 6 years of age:
A total of two (2) doses:
The second dose should be taken at least 1 week (and no more than 6 weeks) after the first dose.
Children 2 to 6 years of age:
A total of three (3) doses:
Each dose should be taken at least 1 week (and no more than 6 weeks) apart. - Booster Dose:
If there is an on-going risk of cholera or in the case of repeated travel an extra (booster) dose is recommended:
Adults and children over 6 years of age:
One (1) booster dose is recommended up to 2 years after the primary course. If more than 2 years have passed, the primary course should be repeated.
Children 2 to 6 years of age:
One (1) booster dose is recommended up to 6 months after the primary course. If more than 6 months have passed, the primary course should be repeated. - If you do not understand the instructions in this leaflet or on the box, ask your doctor or pharmacist for help.
When to take Dukoral®
For best protection against cholera the last dose of vaccine should be taken at least 2 weeks before arrival at your destination.
Each dose should be taken at least 1 week (and no more than 6 weeks) apart.
Do not take doses less than 1 week apart.
How to take Dukoral®
Please follow the instructions and diagrams below:
Avoid food and drink for 1 hour before and 1 hour after vaccination. Food and drink during this time may inactivate the vaccine.
- Dissolve the effervescent powder from the buffer sachet in a glass of cool water (approx. 150 mL).
This is the buffer solution.
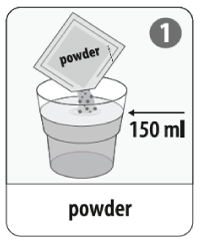
If you are preparing the vaccine for a child 2-6 years of age: pour away (discard) half of the buffer solution leaving approximately 75 mL.
- Shake the vaccine vial gently (1 vial = 1 dose)
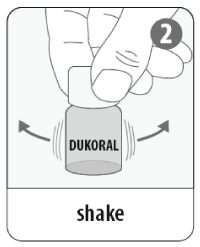
- Add the entire contents of the vaccine vial to the buffer solution. Mix well.
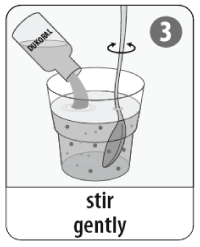
- The mixture should be drunk within 2 hours.
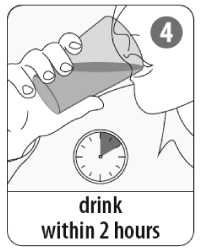
The vaccine must not be injected.
Do not use any liquid except water to make the buffer solution.
it is important to follow these instructions otherwise the vaccine will not work.
If you forget to take Dukoral®
If more than 6 weeks have passed between doses you should talk to your doctor or pharmacist. You may need to repeat the primary course.
If you are not sure what to do, ask your doctor or pharmacist.
If you take too much Dukoral®
If you think that you or anyone else may have used too much Dukoral®, you may need urgent medical attention.
You should immediately:
- phone the Poisons Information Centre
(by calling 13 11 26), or - contact your doctor, or
- go to the Emergency Department at your nearest hospital.
You should do this even if there are no signs of discomfort or poisoning.
Urgent medical attention may be required.
As each vial of Dukoral® contains only one dose, overdosage is unlikely.
| 5. What should I know while taking Dukoral®? |
Things you or your child should do
- Keep an updated record of your vaccinations.
- Keep follow-up appointments with your doctor or clinic.
It is important to take your follow-up doses of Dukoral® at the appropriate times to make sure the vaccine has the best chance of providing protection against cholera.
Remind any doctor, dentist or pharmacist you visit that you are using Dukoral®.
Things you or your child should not do
- Do not have food or drink for 1 hour before and 1 hour after taking Dukoral®.
Food and drink taken during this time may inactivate the vaccine. - Do not have an oral typhoid vaccine for 8 hours after taking Dukoral®.
- Do not have any other oral medicines for 1 hour after taking Dukoral®.
Things to be careful of
Dukoral® is not a sole measure of prevention against cholera. Clean hygiene practices are still required.
Looking after your medicine
Follow the instructions in the carton on how to take care of your medicine properly.
Store it in a refrigerator, between 2°C and 8°C.
Do not freeze Dukoral®.
Freezing destroys the vaccine.
Keep it where young children cannot reach it.
When to discard your medicine
Do not take Dukoral® if the packaging is torn or shows signs of tampering.
Do not take Dukoral® after the expiry date printed on the pack.
Getting rid of any unwanted medicine
If you no longer need to use this medicine or it is out of date, take it to any pharmacy for safe disposal.
Do not use this medicine after the expiry date.
| 6. Are there any side effects? |
All medicines can have side effects. If you do experience any side effects, most of them are minor and temporary. However, some side effects may need medical attention.
See the information below and, if you need to, ask your doctor or pharmacist if you have any further questions about side effects.
Tell your doctor or pharmacist as soon as possible if you, or your child, do not feel well after having Dukoral®.
Less serious side effects
| Less serious side effects | What to do |
| Speak to your doctor, nurse or pharmacist if you or your child have any of these less serious side effects and they worry you. |
Serious side effects
| Serious side effects | What to do |
| Call your doctor straight away, or go straight to the Emergency Department at your nearest hospital if you or your child notice any of these serious side effects. |
Tell your doctor or pharmacist if you notice anything else that may be making you feel unwell.
Other side effects not listed here may occur in some people.
Do not be alarmed by this list of possible side effects.
You or your child may not experience any of them.
Reporting side effects
After you have received medical advice for any side effects you experience, you can report side effects to the Therapeutic Goods Administration online at www.tga.gov.au/reporting-problems. By reporting side effects, you can help provide more information on the safety of this medicine.
Always make sure you speak to your doctor or pharmacist before you decide to stop taking any of your medicines.
| 7. Product details |
This medicine is only available with a doctor's prescription.
What Dukoral® contains
| Active ingredients (main ingredients) |
|
| Other ingredients (inactive ingredients) |
|
| Buffer sachet contains |
Dukoral® does not contain lactose, sucrose, gluten, tartrazine or any other azo dyes. |
Do not take this medicine if you are allergic to any of these ingredients.
What Dukoral looks like
Dukoral® is supplied as a small amount of whitish liquid in a glass vial. The vial is closed with a rubber stopper and a screw cap.
Each vial contains one dose of the vaccine.
Also supplied is one sachet of effervescent powder (buffer). The buffer is a dry powder which is white to off-white in colour.
Dukoral® may be supplied in a carton that contains:
One Dose: 1 vaccine vial and 1 sachet of buffer
Two Doses: 2 vaccine vials and 2 sachets of buffer
Aust R 94483.
Who distributes Dukoral
Seqirus Pty Ltd
ABN 26 160 735 035
63 Poplar Road
Parkville, VIC 3052
Australia
Telephone: 1800 642 865
www.seqirus.com.au
This leaflet was prepared in June 2022.
Dukoral® is a registered trademark of Valneva Sweden AB
Published by MIMS August 2022
