What is in this leaflet
This leaflet answers some common questions about Efexor-XR.
It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking Efexor-XR against the benefits it is expected to have for you.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine. You may need to read it again.
What Efexor-XR is used for
What it does
Efexor-XR is used in the treatment and prevention of relapse of depression. It is also used in the treatment of panic attacks and anxiety, including avoidance or fear of social situations.
Depression can affect your whole body and may cause emotional and physical symptoms such as feeling low in spirit, being unable to enjoy life, poor appetite or overeating, disturbed sleep, loss of sex drive, lack of energy and feeling guilty over nothing.
Excessive anxiety is a condition in which you feel constantly and uncontrollably worried and distressed. It may also make you feel irritable, and cause difficulty in thinking and sleeping. Other common symptoms associated with anxiety may include a dry mouth, a lump in the throat, cold clammy hands, diarrhoea and nausea.
Depression and anxiety are treatable illnesses. Anxiety or tension associated with the normal stress of everyday life usually does not require treatment with medicines.
Ask your doctor if you have any questions about why this medicine has been prescribed for you. Your doctor may have prescribed it for another reason.
How it works
Efexor-XR contains the active ingredient called venlafaxine hydrochloride. It belongs to a class of medications for depression and anxiety, called Serotonin-Noradrenaline Reuptake Inhibitors (SNRIs).
Serotonin and noradrenaline are chemical messengers that allow certain nerves in the brain to work. Efexor-XR capsules increase the level of these two messengers. Experts think this is how it helps to restore your feeling of wellness.
Efexor-XR is not addictive.
It is available only with a doctor's prescription.
Use in Children
Do not give Efexor-XR to children or adolescents under 18 years of age. The safety and effectiveness of Efexor-XR in this age group have not been established.
Before you take Efexor-XR
When you must not take it
Do not take Efexor-XR if you are taking other medications for depression known as monoamine oxidase inhibitors, even if you have stopped taking them now, but have taken them within the last 14 days.
Do not take Efexor-XR if you are allergic to it or to any of the ingredients listed at the end of this leaflet.
Symptoms of an allergic reaction may include:
- Rash, itching or hives on the skin
- Swelling of the face, lips, tongue, throat or other parts of the body
- Shortness of breath, wheezing or troubled breathing; difficulty swallowing.
Do not take this medicine after the expiry date (EXP) printed on the pack or if the packaging is torn or shows signs of tampering. If it has expired or is damaged, return it to your pharmacist for disposal.
If you are not sure whether you should start taking this medicine, talk to your doctor.
Before you start to take it
Tell your doctor or pharmacist if you have allergies to any other medicines, foods, preservatives or dyes.
Tell your doctor or pharmacist if you are pregnant or intend to become pregnant. Efexor-XR is not recommended for use during pregnancy. Your doctor will discuss the risks and benefits of using this medicine if you are pregnant. One of these risks is that newborn babies, whose mothers have been taking Efexor-XR in the last few months of pregnancy, may experience problems soon after delivery, including breathing difficulties, seizures and lack of oxygen in their blood.
If you take Efexor-XR or similar anti-depressants mid to late in your pregnancy, you may develop a condition known as "pre-eclampsia", which is characterised by persistent high blood pressure during or after pregnancy. Symptoms of pre-eclampsia can include headaches, abdominal pain, shortness of breath or burning behind the sternum, nausea and vomiting, confusion, heightened state of anxiety, and/or visual disturbances such as oversensitivity to light, blurred vision, or seeing flashing spots or auras.
If you take Efexor-XR or similar antidepressants in the last month of your pregnancy, you may experience heavy bleeding during and/or after delivery.
Continuing treatment with Efexor-XR or similar antidepressants during pregnancy should be strictly as directed by your doctor. Symptoms of a relapse may occur if treatment is discontinued, even if major depression was previously under control.
Tell your doctor or pharmacist if you are breast-feeding or planning to breast-feed. Efexor-XR passes into breast milk and there is a possibility that the breast-fed baby may be affected. For this reason, the use of Efexor-XR is not recommended in breast-feeding women.
Tell your doctor if you have or have had any of the following medical conditions:
- A history of fits (seizures or convulsions)
- A personal history or family history of bipolar disorder
- A history of aggression
- A history of restlessness or difficulty sitting still
- Diabetes
- Blood pressure problems
- Glaucoma (increased pressure in the eye)
- A tendency to bleed more than normal or you are taking medicines to prevent blood clots
- Raised cholesterol levels or you are taking medicines to lower cholesterol
- Problems with your kidneys or liver
- Problems with your heart, especially conditions causing irregular heartbeats.
Your doctor may wish to do some heart tests such as an electrocardiogram (ECG) or blood tests during treatment with Efexor-XR.
Tell your doctor if you plan to have surgery.
If you have not told your doctor about any of the above, tell them before you start taking Efexor-XR.
Taking other medicines
Tell your doctor or pharmacist if you take any other medicines, including:
- all prescription medicines
- medicines for weight loss
- all medicines, vitamins, herbal supplements or natural therapies you buy without a prescription from a pharmacy, supermarket, naturopath or health food shop.
Do not start to take any other medicine while you are taking Efexor-XR, unless it is prescribed or approved by your doctor.
Some medicines may interfere with Efexor-XR, or Efexor-XR may interfere with these medicines. These include:
- Medications for depression known as monoamine oxidase inhibitors (such as moclobemide, linezolid, phenelzine and tranylcypromine), even if you have stopped taking them now, but have taken them within the last 14 days. Your doctor or pharmacist can tell you what to do if you are taking any of these medicines
- Allow at least 7 days after stopping EFEXOR-XR before starting a MAOI
Taking this medicine with a MAOI, or within 7 days of taking a MAOI, may cause a serious reaction with a sudden increase in body temperature, extremely high blood pressure and severe convulsions.
The appropriate washout period is needed to prevent severe adverse reactions. - Any other medications for depression, anxiety, obsessive-compulsive disorder or premenstrual dysphoric disorder, including St John's wort
- Medicines for treating mental disorders such as haloperidol, risperidone, lithium or clozapine
- Tramadol, fentanyl, tapentadol, pethidine and methadone used to treat strong pain
- Medicines used to treat Attention Deficit Hyperactivity Disorder (ADHD) such as dexamphetamine and lisdexamphetamine
- Cimetidine for reflux and stomach ulcers
- Triptans used to treat migraine
- Amiodarone or quinidine used to treat irregular heartbeats.
Your doctor may do some tests such as an electrocardiogram (ECG) or blood tests if you are taking either of these medicines whilst taking Efexor-XR.
- Metoprolol for high blood pressure or angina
- Medicines used to prevent blood clotting such as anti-coagulants and platelet inhibitors
- Indinavir for viral infections
- Antibiotics such as erythromycin and linezolid for bacterial infections
- Ketoconazole or fluconazole for fungal infections.
You may need to use different amounts of your medicine, or you may need to take different medicines. Your doctor will advise you.
Your doctor and pharmacist have more information on medicines to be careful with or avoid while this medicine.
How to take Efexor-XR
Follow all directions given to you by your doctor or pharmacist carefully. They may differ from the information contained in this leaflet.
If you do not understand the instructions on the box, ask your doctor or pharmacist for help.
How much to take
Your doctor will tell you how many capsules you need to take each day. This may depend on your age, your condition and whether or not you are taking any other medicines.
Depression and Anxiety
The usual starting dose is 75mg taken once daily. After 2 weeks, your doctor may increase the dose to 150 mg a day.
Panic attacks
The usual starting dose is 37.5 mg taken once daily for the first 4 to 7 days, then increased to 75 mg taken once daily.
Do not change your dose unless your doctor tells you to. If you have kidney or liver problems, you may need a lower dose of Efexor-XR.
If you have heart problems your doctor may first do some blood tests or heart tests such as an electrocardiogram (ECG) before increasing your dose of Efexor XR.
How to take it
Swallow the capsules whole with a glass of water or other non-alcoholic liquid.
Do not divide, crush, chew or dissolve the capsules in water.
Do not be concerned if you see small white granules or balls in your stools after taking Efexor-XR. Inside Efexor-XR capsules are spheroids or small white balls that contain the venlafaxine active ingredient. These spheroids are released from the capsule into your gastrointestinal tract. As the spheroids travel the length of your gastrointestinal tract, venlafaxine is slowly released. The spheroid 'shell' remains undissolved and is eliminated in your stools. Therefore, even though, you may see spheroids in your stools, your dose of venlafaxine has been absorbed.
When to take it
Take your medicine once daily with food, at approximately the same time each day. This could be either in the morning or in the evening.
Taking it at the same time each day will have the best effect. It will also help you remember when to take it.
Avoid drinking alcohol while you are taking Efexor-XR.
How long to take it
Continue taking your medicine for as long as your doctor tells you. Although you may begin to feel better after two weeks, it may take several weeks before you feel much better. It is important to give Efexor-XR time to work.
This medicine helps to control your condition, but does not cure it. It is important to keep taking your medicine even if you feel well.
If you forget to take it
If it is less than 12 hours until your next dose, skip the dose you missed and then take your next dose when you are meant to. Otherwise, take it as soon as you remember, and then go back to taking as you would normally.
Do not take a double dose to make up for the dose you missed. This may increase the chance of you getting an unwanted side effect.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering to take your medicine, ask your pharmacist for some hints.
If you take too much (overdose)
Immediately telephone your doctor or Poisons Information Centre (Tel Australia 13 11 26) for advice, or go to Accident and Emergency at your nearest hospital if you think that you or anyone else may have taken too much Efexor-XR.
Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
If you take too many Efexor-XR capsules you may:
- Feel sleepy
- Vomit
- Have an increased heart rate or changes in heart rhythm
- Have a seizure (fits)
- Have breathing difficulties
- Become unconscious
- Have dilated pupils.
Keep the telephone number for these places handy whilst taking any medications.
While you are taking Efexor-XR
Things you must do
Visit your doctor regularly for a check up so that your progress can be checked. Your doctor may do some tests (such as an electrocardiogram (ECG) or blood tests) from time to time to make sure the medicine is working and to prevent unwanted side effects.
Always discuss any questions you have about Efexor-XR capsules with your doctor.
If you are going to have surgery, tell your doctor that you are taking this medicine. Some agents used to assist your doctor during surgery may interact with Efexor-XR leading to unwanted side effects.
If you are about to have any urine tests, tell your doctor that you are taking this medicine. It may interfere with the results of some tests.
Take Efexor-XR capsules as your doctor has prescribed.
Keep enough Efexor-XR capsules to last weekends and holidays.
This medicine helps to control your condition, but does not cure it. It is important to keep taking your medicine even if you feel well.
Tell any other doctors, dentists, and pharmacists who treat you that you are taking this medicine.
Watch carefully for signs that your depression or anxiety is getting worse, especially in the first few weeks of treatment, or if your dose has changed. Sometimes people with depression can experience a worsening of their depressive symptoms. This can happen even when taking an antidepressant.
Information from clinical trials has suggested that children, adolescents and young adults (18-24 years), particularly those with depression, may be at increased risk of suicidal behaviour (including suicide attempts) when treated with Efexor-XR, especially during initial treatment.
Tell your doctor immediately if you experience any of the following symptoms, especially if they are severe, you have not had these symptoms before or they happen very suddenly.
- Anxiety or agitation
- Panic attacks
- Difficulty sleeping
- Irritability
- Aggressiveness
- Hostility or impulsiveness
- Restlessness
- Overactivity or uninhibited behaviour
- Other unusual changes in behaviour
- Thoughts of suicide
- Tremor, sweating, fast heart rate
- Muscle rigidity.
Symptoms of serotonin syndrome may include mental status changes (e.g. agitation, confusion, hallucinations, and coma), autonomic instability (e.g. excessive sweating, fast heart rate, and increased body temperature), neuromuscular aberrations (e.g. overactive reflexes, incoordination, tremor) and/or gastrointestinal symptoms (e.g. nausea, vomiting, and diarrhoea).
Tell your doctor immediately if you have any thoughts about suicide or doing harm to yourself.
Warning signs of suicide
If you or someone you know is showing the following warning signs, either contact your doctor or a mental health advisor right away or go to the nearest hospital for treatment.
All thoughts or talk about suicide or violence are serious.
- Thoughts or talk about death or suicide
- Thoughts or talk about self-harm or doing harm to others
- Any recent attempts of self-harm
- An increase in aggressive behaviour, irritability or agitation.
Things to be careful of
Be careful driving or operating dangerous machinery until you know how it affects you. Efexor-XR capsules may make you feel drowsy.
If you are feeling drowsy or are uncoordinated, be careful that you do not fall over.
Efexor-XR, like other medicines in this class, may increase your risk of bone fracture.
Things you must not do
Do not suddenly stop taking Efexor-XR or lower the dose if you have been taking it for some time. If possible, your doctor will gradually reduce the amount you take each day before stopping the medicine completely.
If you stop taking it suddenly, your condition may worsen or you may have unwanted side effects such as:
- Headache
- Nausea and vomiting
- Dizziness
- Insomnia
- Nervousness
- Anxiety
- Confusion and agitation
- Diarrhoea
- Sweating
- Loss of appetite
- Tremor
- Flu-like symptoms
- Impaired coordination and balance
- Tingling or numbness of the hands and feet.
Slowly reducing the amount of Efexor-XR being taken reduces the possibility of these effects occurring.
Some of these symptoms may impair driving, or the operation of dangerous machinery. Avoid these activities if you experience these symptoms.
Do not give this medicine to anyone else even if they have the same condition as you.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking Efexor-XR.
All medicines can have side effects. Sometimes they are serious; often they are not. You may need medical attention if you get some of the side effects.
It can be difficult to tell whether side effects are the result of taking this medicine, effects of your condition, or side effects of other medicines you may be taking. For this reason it is important to tell your doctor of any change in your condition.
Do not be alarmed by the list of side effects. You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor if...
Tell your doctor or pharmacist if you notice any of the following and they worry you:
- Stomach, bowel or urinary tract problems:
- Nausea or vomiting
- Loss of appetite
- Diarrhoea
- Constipation
- Difficulty passing urine, passing urine more frequently, or urinary incontinence - Changes in your behaviour:
- Difficulty sleeping or abnormal dreams
- Paranoia
- Aggression
- Sexual function problems such as delayed ejaculation, problems with erection, decreased sex drive or difficulties achieving orgasm
- Nervousness
- Teeth grinding
- Impaired coordination and balance - Difficulty thinking or working because of:
- Yawning
- Feeling sedated or drowsy
- Fainting or dizziness after standing up
- Restlessness or difficulty sitting still
- Headache
- Rapid heart beat
- Heavy or irregular menstrual periods - Sweating
- Hot flushes
- Rash
- Hair loss
- Itchiness
- Weight loss
- Weight gain
- Flow of milk in women who are not breastfeeding
- Blurred vision
- Ringing in the ears
- Altered taste
- Dry mouth.
Tell your doctor as soon as possible if...
Tell your doctor as soon as possible if you notice any of the following:
- Muscle tremors, spasms, twitching, jerky movements or sustained muscle contractions
- Abnormal facial movements such as tongue thrusting, repetitive chewing, jaw swinging, or grimacing
- A feeling of apathy or not caring about things
- Hallucinations
- Agitation
- Confusion
- Unusually overactive
- Changes in muscle tone, muscle weakness or fatigue
- Numbness or pins and needles.
- Problems with breathing, shortness of breath
- Cough
- Bleeding or bruising more easily than normal
- Sensitivity to sunlight.
Go to hospital if...
Tell your doctor immediately, or go to Accident and Emergency at your nearest hospital if you notice any of the following:
- Fits or seizures, which may be accompanied by a sudden fever
- Signs of allergy such as rash or hives, swelling of the face, lips, tongue or throat, wheezing or difficulty breathing or swallowing
- Symptoms of sudden fever with sweating, rapid heartbeat and muscle stiffness, which may lead to loss of consciousness (symptoms resembling neuroleptic malignant syndrome)
- Palpitations, shortness of breath, intense chest pain, or irregular heartbeats
- Dark, red or cola-coloured urine, muscle weakness and tenderness, stiffness or aching
- Stomach pain, yellowing of the skin, nausea, fever, clammy skin and sweating
- Yellowing of the skin or eyeballs, fever, fatigue, loss of appetite, dark coloured urine or light coloured bowel movements
- A severe skin reaction with painful red areas and large blisters, accompanied by fever and chills, aching muscles and generally feeling unwell
- Symptoms of a high fever, agitation, confusion, trembling and abrupt contractions of muscles
- Signs of an infection such as severe chills, fever, sore throat and mouth ulcers
- Black sticky bowel motions or bloody diarrhoea.
These symptoms are usually rare but may be serious and need urgent medical attention.
Tell your doctor or pharmacist if you notice anything else that is making you feel unwell. Other side effects not listed above may also occur in some people. Some of these side effects (for example, increase in blood pressure or blood cholesterol) can only be found when your doctor does tests from time to time to check your progress.
After taking Efexor-XR
Storage
Keep your capsules in their blister pack until it is time to take them. If you take the capsules out of the blister pack they may not keep well.
Keep Efexor-XR capsules in a cool dry place where the temperature stays below 30°C.
Do not store Efexor-XR capsules or any other medicine in the bathroom or near a sink. Do not leave it in the car or on windowsills. Heat and dampness can destroy some medicines.
Keep it where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking this medicine or the expiry date has passed, ask your pharmacist what to do with any medicine that is left over.
Product description
What it looks like
There are three strengths of Efexor-XR capsules:
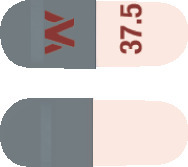
- Efexor-XR 37.5 mg capsules are grey and peach in colour
- Efexor-XR 75 mg capsules are opaque peach in colour
- Efexor-XR 150 mg capsules are opaque dark orange in colour.
Each capsule is printed with a "W" and the capsule strength.
Each blister pack contains 28 capsules.
Ingredients
Efexor-XR contains 37.5 mg, 75 mg and 150 mg venlafaxine hydrochloride as the active ingredient.
It also contains:
- Iron oxide yellow CI 77492
- Iron oxide red CI 77491
- Microcrystalline cellulose
- Ethylcellulose
- Hypromellose
- Gelatin
- Purified talc
- Titanium dioxide
- Red ink Opacode S-1-15094/ S-1-15095 (37.5 mg and 75 mg strengths)
- White ink TekPrint SB-0007P (150 mg strength)
- Iron oxide black CI 77499 (Efexor-XR 37.5 mg only).
Efexor-XR does not contain gluten, sucrose, tartrazine or any other azo dyes.
Supplier
Efexor-XR capsules are supplied in Australia by:
Viatris Pty Ltd
Level 1, 30 The Bond
30-34 Hickson Road
Millers Point NSW 2000
www.viatris.com.au
Phone: 1800 274 276
Australian registration numbers
37.5 mg capsule: AUST R 99802
75 mg capsule: AUST R 60858
150 mg capsule: AUST R 60859
Date of preparation
This leaflet was prepared in November 2022.
EFEXOR® is a Viatris company trade mark
ujcefexc11122
Published by MIMS January 2023