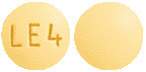Notes
Distributed by Arrotex Pharmaceuticals
1 Name of Medicine
Levonorgestrel and ethinylestradiol.
2 Qualitative and Quantitative Composition
Eleanor 150/30 ED is a combined oral contraceptive (COC) tablet containing the synthetic progestogen levonorgestrel and the synthetic estrogen ethinylestradiol.
Each yellow film-coated active tablet contains 30 microgram ethinylestradiol and 150 microgram levonorgestrel.
List of excipients with known effects: Lactose monohydrate.
For the full list of excipients, see Section 6.1 List of Excipients.
3 Pharmaceutical Form
Active tablet. Yellow, round biconvex film-coated tablets debossed with LE4 on one side and plain on other side.
Placebo tablet. White to off-white, round shaped, biconvex film-coated tablets, plain on both sides.
4 Clinical Particulars
4.9 Overdose
There have been no reports of serious deleterious effects from overdose. Symptoms that may occur in this case are: nausea, vomiting and withdrawal bleeding. The last may even occur in girls before their menarche, if they have accidentally taken the medicinal product. There are no antidotes and further treatment should be symptomatic.
For information on the management of overdose, contact the Poisons Information Centre on 13 11 26 (Australia).
5 Pharmacological Properties
5.3 Preclinical Safety Data
Genotoxicity. There is limited evidence available in the literature suggesting that estrogens may be weakly genotoxic at high doses. Ethinylestradiol was negative in studies for DNA-adduct formation in cultured human liver slices and in assays for gene mutations (bacterial or mammalian cells in vitro) and gave equivocal results in assays for chromosomal damage (clastogenic effects were not consistently seen and occurred at high doses).
The genotoxic potential of levonorgestrel has not been fully investigated, although limited data available to date suggest that it does not appear to be genotoxic.
Carcinogenicity. Long-term continuous administration of natural and synthetic estrogens in certain animal species increases the frequency of carcinomas of the breast, uterus, cervix, vagina, testis and liver. A long-term study with levonorgestrel in dogs showed an increased incidence of mammary tumours, although a similar effect was not apparent in studies in mice, rats or monkeys. The occurrence of these mammary tumours in dogs may be due in part to a hormonal feedback mechanism. The clinical relevance of these findings is uncertain.
Numerous epidemiological studies have been conducted to determine the incidence of breast, endometrial, ovarian and cervical cancer in women taking COCs. Some of these studies have shown an increased relative risk of breast cancer in certain subgroups of COC users. Women with a strong family history of breast cancer or who have breast nodules, fibrocystic disease or abnormal mammograms should be monitored with particular care. Benign hepatic adenomas have been found to be associated with the use of oral contraceptives. Although benign, hepatic adenomas may rupture and cause death through intra-abdominal haemorrhage. Some epidemiological studies also suggest that COC use has been associated with an increase in the risk of cervical intraepithelial neoplasia in some populations of women, although there continues to be controversy about the extent to which this finding is attributable to the confounding effects of sexual behaviour and other factors such as HPV. It must also be borne in mind that sexual steroids can promote the growth of certain hormone dependent tissues and tumours (see Section 4.4 Special Warnings and Precautions for Use).
6 Pharmaceutical Particulars
6.7 Physicochemical Properties
Levonorgestrel is a white or almost white, crystalline powder. Practically insoluble in water; sparingly soluble in methylene chloride and slightly soluble in ethanol (96%).
Ethinylestradiol is a white or slightly yellowish-white crystalline powder. It is practically insoluble in water, soluble in chloroform and in ether, freely soluble in alcohol.
Chemical structure. Levonorgestrel.
https://stagingapi.mims.com/au/public/v2/images/fullchemgif/CSLEVONO.gif Chemical name: 13β-ethyl-17β-hydroxy-18, 19-dinor-17α-pregn-4-en-20-yn-3-one.
Molecular formula: C21H28O2.
Molecular weight: 312.45.
Ethinylestradiol.
https://stagingapi.mims.com/au/public/v2/images/fullchemgif/CSETHEST.gif Chemical name: 19-nor-17α-pregna-1,3,5(10)-trien-20-yne-3, 17β-diol.
Molecular formula: C20H24O2.
Molecular weight: 296.41.
CAS number. Levonorgestrel. 797-63-7.
Ethinylestradiol. 57-63-6.
7 Medicine Schedule (Poisons Standard)
(S4) Prescription Only Medicine.
Summary Table of Changes
https://stagingapi.mims.com/au/public/v2/images/fulltablegif/ELEANOST.gif
References
1. Dinger JC, Heinemann LA, Kuhl-Habich D. The safety of a drospirenone-containing oral contraceptive: final results from the European Active Surveillance study on Oral Contraceptives based on 142,475 women-years of observation. Contracept 2007; 75:344-54.
2. Long-term Active Surveillance Study for Oral contraceptives (LASS), 2nd update report based on study status. May 2009.