What is in this leaflet
What Estrofem® is used for
Before you take Estrofem®
How to take Estrofem®
While you are taking Estrofem®
Side effects
Storage
Product Description
User instructions
This leaflet answers some common questions about Estrofem®. It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you using Estrofem® against the benefits they expect it will have for you.
Ask your doctor or pharmacist if you have any concerns about using this medicine.
Keep this leaflet with the medicine. You may need to read it again.
Estrofem® is available only by prescription at pharmacies.
What Estrofem® is used for
Estrofem® is a hormone replacement therapy (HRT) that is used for the short-term symptomatic treatment of women who have had their womb surgically removed (which is called hysterectomy) and who have signs and symptoms of estrogen deficiency.
A woman’s last menstrual period is called the menopause and usually occurs between the ages of 45 and 55 years. Around the time of the menopause, the body slowly stops producing the two sex hormones called estrogen and progesterone. Periods become irregular until they finally stop.
If a woman has surgical or natural menopause the production of these hormones is diminished or stopped.
Estrofem® replaces the hormone called estrogen which your body stops making after surgical or natural menopause.
The falling or reduced hormone levels may cause you to experience uncomfortable symptoms such as hot flushes, night sweats, sleeplessness, dry vagina, urinary problems, headaches, mood swings, lack of concentration or loss of energy.
The estrogen in Estrofem® relieves the symptoms caused by a lack of estrogen.
If you have not had a hysterectomy your doctor may prescribe another medicine (a progesterone type medicine called a ‘progestagen’) to be taken with Estrofem® for 10-14 days of your 28 day cycle. It is very important to take both medications exactly the way your doctor has prescribed.
If you have any questions about the combination of the two medicines please talk to your doctor.
Your doctor may have prescribed Estrofem® for another reason. Ask your doctor if you have any questions about why Estrofem® has been prescribed for you.
Before you take Estrofem®
When you must not take it
Do not take Estrofem® if:
- you are pregnant or suspect you may be pregnant, or you are breast-feeding
- you know or suspect you have, or you have had, a tumour which depends on hormones (e.g. cancer of the breast or the lining of the womb)
- you know or suspect you have, or you have had, breast cancer
- you have recently developed liver disease; or you have a history of liver disease where your blood test results have not returned to normal
- you have porphyria (a rare disease of blood pigments)
- you have any unexplained vaginal bleeding
- you have excessive thickening of the womb lining (endometrial hyperplasia) that is not being treated
- you know that you are allergic to any of the ingredients in Estrofem®
- you have or ever had any kind of blood clots
- you have a blood clotting disorder (such as protein C, protein S, or antithrombin deficiency)
- you have pain in your calf and your leg is swelling
- it is after the expiry date (‘Expiry’) printed on the pack
- the packaging is torn or shows signs of tampering
Before you start to take it
Medical check-ups
Before you start taking HRT, your doctor should ask about your own and your family’s medical history.
Your doctor may decide to examine your breasts and/or your abdomen, and may do an internal examination - but only if these examinations are necessary for you, or if you have any special concerns.
Once you’ve started on HRT, you should see your doctor for regular check-ups (at least once a year). At these check-ups, your doctor may discuss with you the benefits and risks of continuing to take HRT.
Be sure to:
- go for regular breast screening and pap smear tests
- regularly check your breasts for any changes such as dimpling of the skin, changes in the nipple, or any lumps you can see or feel
There is only limited experience of treating women older than 65 years with Estrofem®.
Tell your doctor if:
- you have not had a hysterectomy, because another medicine may be more suitable for you
- you have previously taken estrogen by itself for menopausal symptoms and have not had a hysterectomy. The long-term use of estrogen without a progesterone can increase the risk of cancer of the lining of the womb
- you have a family history of blood clots
- you are to be hospitalised or undergoing surgery, particularly where you are or will be off your feet for a long time. You may need to stop taking Estrofem® 4 to 6 weeks before your operation, to reduce the risk of a blood clot
- you have an intolerance to some specific sugars e.g. lactose (found in milk and milk products). Estrofem® tablets contain lactose
Tell your doctor if you have or have had any medical conditions, especially the following:
- fibroids of the womb or endometriosis and you have not had a hysterectomy. Fibroids may increase in size while taking Estrofem® and symptoms of endometriosis may worsen
- a history of excessive growth of the cells which line the womb
- family history of hormone-dependent cancer e.g. cancer of the breast or of the lining of the womb
- increased risk of developing blood clots (see Blood clots in a vein (thrombosis))
- high blood pressure
- inflammation of the pancreas due to high levels of blood fats (triglycerides)
- hereditary or acquired angioedema
- systemic lupus erythematosus (SLE)
- epilepsy, migraine, severe headache
- diabetes
- asthma
- gallstones
- a liver disorder, such as a benign liver tumour
- heart disease
- kidney disease
- fluid retention (oedema)
- otosclerosis (hearing loss sometimes linked to pregnancy)
This is because you will need to be seen regularly by your doctor while you are taking Estrofem®.
HRT and cancer
Endometrial hyperplasia endometrial cancer)
Taking estrogen-only HRT will increase the risk of excessive thickening of the lining of the womb (endometrial hyperplasia) and cancer of the womb lining (endometrial cancer).
Taking a progestagen in addition to the estrogen for at least 10 days of each 28 day cycle protects you from this extra risk. So your doctor will prescribe a progestagen separately if you still have your womb. If you have had your womb removed (a hysterectomy), discuss with your doctor whether you can safely take this product without a progestagen.
Compare
In women who still have a womb and who are not taking HRT, on average, 5 in 1,000 will be diagnosed with endometrial cancer between the ages of 50 and 65.
For women aged 50 to 65 who still have a womb and who take estrogen-only HRT, between 10 and 60 women in 1,000 will be diagnosed with endometrial cancer (i.e. between 5 and 55 extra cases), depending on the dose and for how long it is taken.
Unexpected bleeding
You will have a bleed once a month (so-called withdrawal bleed) while taking Estrofem®.
See your doctor as soon as possible if you have not had a hysterectomy and you have unexpected bleeding or drops of blood (spotting) besides your monthly bleeding, which:
- carries on for more than the first 6 months, or
- starts after you have been taking Estrofem® more than 6 months, or
- carries on after you have stopped taking Estrofem®.
Breast cancer
Evidence shows that taking combined estrogen-progestagen or estrogen-only hormone replacement therapy (HRT) increases the risk of breast cancer. The extra risk depends on how long you use HRT. The additional risk becomes clear within 3 years of use. After stopping HRT the extra risk will decrease with time, but the risk may persist for 10 years or more if you have used HRT for more than 5 years.
Compare
Women aged 50 to 54 who are not taking HRT, on average, 13 to 17 in 1,000 will be diagnosed with breast cancer over a 5-year period.
For women aged 50 who start taking estrogen-only HRT for 5 years, there will be 16-17 cases in 1,000 users (i.e. an extra 0 to 3 cases).
For women aged 50 who start taking estrogen-progestagen HRT for 5 years, there will be 21 cases in 1,000 users (i.e. an extra 4 to 8 cases).
Women aged 50 to 59 who are not taking HRT, on average, 27 in 1,000 will be diagnosed with breast cancer over a 10-year period.
For women aged 50 who start taking estrogen-only HRT for 10 years, there will be 34 cases in 1,000 users (i.e. an extra 7 cases).
For women aged 50 who start taking estrogen-progestagen HRT for 10 years, there will be 48 cases in 1,000 users (i.e. an extra 21 cases).
Regularly check your breasts. See your doctor if you notice any changes such as:
- dimpling of the skin
- changes in the nipple
- any lumps you can see or feel.
Additionally, you are advised to join mammography screening programs when offered to you. For mammogram screening, it is important that you inform the nurse/healthcare professional who is actually taking the x-ray that you use HRT, as this medication may increase the density of your breasts which may affect the outcome of the mammogram. Where the density of the breast is increased, mammography may not detect all lumps.
Ovarian cancer
Ovarian cancer is rare - much rarer than breast cancer. The use of estrogen-only or combined estrogen-progestagen HRT has been associated with a slightly increased risk of ovarian cancer.
The risk of ovarian cancer varies with age. For example, in women aged 50 to 54 who are not taking HRT, about 2 women in 2,000 will be diagnosed with ovarian cancer over a 5-year period. For women who have been taking HRT for 5 years, there will be about 3 cases per 2,000 users (i.e. about 1 extra case).
Effect of HRT on heart and circulation
Blood clots in a vein (thrombosis)
The risk of blood clots in the veins is about 1.3- to 3-times higher in HRT users than in non-users, especially during the first year of taking it.
Blood clots can be serious, and if one travels to the lungs, it can cause chest pain, breathlessness, fainting or even death.
You are more likely to get a blood clot in your veins as you get older and if any of the following applies to you.
Inform your doctor if any of these situations applies to you:
- you are unable to walk for a long time because of major surgery, injury or illness (see also ‘If you need to have surgery’)
- you are seriously overweight (BMI >30 kg/m²)
- you have any blood clotting problem that needs long-term treatment with a medicine used to prevent blood clots
- if any of your close relatives has ever had a blood clot in the leg, lung or another organ
- you have systemic lupus erythematosus (SLE)
- you have cancer.
For signs of a blood clot, see ‘When you must not take it’.
Compare
Looking at women in their 50s who are not taking HRT, on average, over a 5-year period, 4 to 7 in 1,000 would be expected to get a blood clot in a vein.
For women in their 50s who have been taking estrogen-progestagen HRT for over 5 years, there will be 9 to 12 cases in 1,000 users (i.e. 5 extra cases).
For women in their 50s who have had their womb removed and have been taking estrogen-only HRT for over 5 years, there will be 5 to 8 cases in 1,000 users (i.e. 1 extra case).
Heart disease (heart attack)
There is no evidence that HRT will prevent a heart attack.
Women over the age of 60 years who use estrogen-progestagen HRT are slightly more likely to develop heart disease than those not taking any HRT.
For women who have had their womb removed and are taking estrogen-only therapy there is no increased risk of developing a heart disease.
Stroke
The risk of getting stroke is about 1.5-times higher in HRT users than in non-users. The number of extra cases of stroke due to use of HRT will increase with age.
Compare
Looking at women in their 50s who are not taking HRT, on average, 8 in 1,000 would be expected to have a stroke over a 5-year period. For women in their 50s who are taking HRT, there will be 11 cases in 1,000 users, over 5 years (i.e. 3 extra cases).
Other conditions
HRT will not prevent memory loss. There is some evidence of a higher risk of memory loss in women who start using HRT after the age of 65. Talk to your doctor for advice.
Tell your doctor immediately if any of the following conditions occur (because you may be told to stop taking Estrofem®):
- yellowing of your skin or eyes, or worsening of your liver function
- significant increase in your blood pressure
- migraine-like headache, and you have not previously had migraines
- sudden development of visual problems
- pregnancy
- or if you experience any of the conditions listed above under ‘When you must not take it’
If you have not told your doctor about any of the above, tell them before you take Estrofem®. Estrofem® should only be used to treat symptoms of the menopause that adversely affect your quality of life.
Review the risks and benefits of continued treatment with Estrofem® at least once a year with your doctor.
Taking other medicines
Tell your doctor if you are taking or plan to take other medicines, including:
- some medicines to help you sleep, including barbiturates
- some medicines for epilepsy e.g. phenytoin and carbamazepine
- some antibiotics and other anti-infective medicines e.g. rifampicin, rifabutin, nevirapine, efavirenz
- some anti-infectives such as ritonavir and nelfinavir, when used at the same time as steroid hormones
- St. John’s Wort - used to treat depression
Tell your doctor if you are taking any other medicines, including any that you buy without a prescription from your pharmacy, supermarket or health food shop. The effect of Estrofem® can be reduced by other medicines, and may affect your vaginal bleeding pattern.
How to take Estrofem®
How to take it
Read carefully the instructions included in this leaflet, in order to correctly use the calendar pack.
Take one tablet a day, preferably at the same time each day, until all 28 tablets have been taken. Swallow each tablet with a glass of water. When you have finished each pack, start the next pack immediately.
If you are not on any other hormone replacement therapy and you have had a hysterectomy you can start taking Estrofem® on any day that is convenient. If you are still experiencing bleeds, you should start using Estrofem® on day 5.
Estrofem® should not be taken by children, men, during pregnancy or while breast-feeding.
Duration of therapy:
HRT should be prescribed at the lowest effective dose and for the shortest duration necessary (see ‘Before you start to take it’). The continuation of the treatment should be re-evaluated annually. Women who have undergone a premature menopause (e.g. hysterectomy) may require longer term treatment.
If you forget to take it
If you forget to take your tablet at the usual time, take it within the next 12 hours. If more than 12 hours have gone by, skip the missed dose and start again as normal the next day. Do not take a double dose to make up for a forgotten tablet. Forgetting a dose may increase the likelihood of breakthrough bleeding and spotting if you still have your womb.
If you take too much (overdose)
If you take more tablets than you have been prescribed, contact your doctor for advice. Overdose may cause nausea and vomiting.
If you need to have surgery
If you are going to have surgery, tell the surgeon that you are taking Estrofem®. You may need to stop taking Estrofem® about 4 to 6 weeks before the operation to reduce the risk of a blood clot (see ‘Blood clots in a vein’).
Ask your doctor when you can start taking Estrofem® again.
While you are taking Estrofem®
You can expect your symptoms to improve within a few months of starting Estrofem®.
If you get breakthrough bleeding or spotting, it is usually nothing to worry about, especially during the first few months of taking HRT (see also ‘Before you start to take it - Unexpected bleeding’ for more information).
Estrofem® can be stopped at any time. You should discuss this with your doctor.
Estrofem® is not a contraceptive and will not prevent pregnancy. Estrofem® is only recommended for women who have signs and symptoms of estrogen deficiency due to surgical or natural menopause and who have had a hysterectomy performed. At your routine check-up, your doctor may reassess your continued need for Estrofem®. Alternative HRT treatment may be given if troublesome symptoms remain.
If you have any concerns about taking Estrofem®, ask your doctor or pharmacist. If your doctor tells you to stop taking Estrofem®, return any unused medicine to your pharmacist.
Things you must not do
This medicine is for you only. Do not give it to someone else even if they seem to have the same symptoms as you.
Do not take Estrofem® to treat any other complaints unless your doctor tells you to.
Do not change the way you take Estrofem®, or lower the dosage, without checking with your doctor.
Side effects
All medicines can have side effects. Sometimes they are serious, most of the time they are not.
Tell your doctor or pharmacist if you experience any side effects while you are taking Estrofem® (whether or not they are mentioned below). You may need medical treatment if you experience some of the side effects.
When you start taking Estrofem® your body has to adjust to new hormone levels. You may experience the following side effects:
- abdominal (stomach) pain, feeling sick (nausea), vomiting, diarrhoea, bloating, flatulence, indigestion
- skin rash or itching, skin reactions, changes in hair growth
- headache, migraine, epilepsy, dizziness
- changes in libido, problems getting to sleep
- breast tenderness, enlargement or pain
- asthma, or worsening of asthma
- gall bladder problems
- leg cramps
- weight increase
- fluid retention (oedema)
- swelling under the skin (angioedema)
- fungal infection of the vagina (thrush)
These side effects are usually temporary and disappear.
Tell your doctor if:
- you think you may be suffering from depression
- you are not feeling well or find any side effect too uncomfortable or unacceptable
- any side effect becomes worse
- you have not had a hysterectomy - vaginal bleeding or spotting suddenly becomes heavier
Tell your doctor immediately if any of the following conditions occur (because you may be told to stop taking Estrofem®):
- pain in your calf and your leg is swelling
- any kind of blood clots
- yellow colouring of the skin and eyes (jaundice)
- migraine or sudden severe headache, and you have not previously had migraines
- problems with your eyesight which develop suddenly
- marked rise in blood pressure
- if you have not had a hysterectomy - vaginal bleeding or spotting occurring after you have been period-free for some time
- you can see or feel a lump in your breast, or you notice dimpling of the skin or changes in the nipple
- you know or suspect you are pregnant
Tell your doctor immediately or go to Accident and Emergency at your nearest hospital if you notice any of the following:
- skin rashes over a large part of the body
- shortness of breath, wheezing
- swelling of the face, lips or tongue
- fast pulse
- sweating
This list includes very serious side effects. You may need urgent medical attention or hospitalisation. These side effects are very rare.
In addition to the possible side effects listed above, ovarian cancer, heart disease, stroke and dementia have been reported with HRT.
Do not be alarmed by these lists of possible side effects. You may not experience any of them.
Storage
Keep all medicines out of reach of children.
Do not use Estrofem® after the expiry date stated on the carton and label. The expiry date refers to the last day of that month.
Keep Estrofem® in a cool dry dark place where the temperature stays below 25°C. Do not put Estrofem® in the refrigerator.
Do not dispose of medicines down the sink or in your household rubbish. Ask you pharmacist how to dispose of medicines you no longer require. These measures will help to preserve the environment.
Product Description
What Estrofem® looks like
Estrofem® comes in a calendar dial pack. Each pack holds 28 round tablets.
Estrofem® 1 mg tablets are red, film-coated, round, biconvex, and marked ‘NOVO 282’ on one side. Estrofem® 2 mg tablets are blue, film-coated, round, biconvex, and marked ‘NOVO 280’ on one side.
Ingredients
Each Estrofem® 1 mg tablet contains 1 mg estradiol (as hemihydrate) as the active ingredient and iron oxide red as a colouring agent.
Each Estrofem® 2 mg tablet contains 2 mg estradiol (as hemihydrate) as the active ingredient and indigo carmine as a colouring agent.
Estradiol is identical to natural human estrogen.
The tablets also contain lactose, hyprolose, maize starch, purified talc, propylene glycol (red 1 mg tablets), Macrogol 400 (blue 2 mg tablets), magnesium stearate, hypromellose and titanium dioxide.
Estrofem® is gluten-free.
Manufacturer
Estrofem® is supplied in Australia by:
Novo Nordisk Pharmaceuticals Pty Ltd.
Level 10
118 Mount Street
North Sydney NSW 2060
Australian Registration Numbers:
Estrofem® 1 mg AUST R 188520
Estrofem® 2 mg AUST R 188521
Estrofem® is a registered trademark of Novo Nordisk Healthcare AG.
© 2023
Novo Nordisk A/S
Always check the following websites to ensure you are reading the most recent version of the Estrofem® consumer medicine information:
www.novonordisk.com.au
https://www.ebs.tga.gov.au/
For further information call Novo nordisk Customer Care on 1800 668 626.
This leaflet was prepared in October 2023.
User instructions
How to use the calendar dial pack
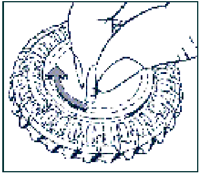
-
Set the day reminder
Turn the inner disc to set the day of the week opposite the little plastic tab.
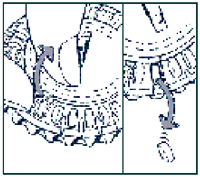
-
Take the first day’s tablet
Break the plastic tab and tip out the first tablet.
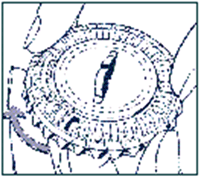
-
Move the dial every day
On the next day, simply move the transparent dial clockwise 1 space as indicated by the arrow. Tip out the next tablet. Remember to take only 1 tablet once a day.
You can only turn the transparent dial after the tablet in the opening has been removed.
Published by MIMS January 2024

