What is in this leaflet
This leaflet answers some common questions about Evogam®.
It does not contain all the available information. If you require further information about this medicine or your treatment, have any questions, or are not sure about something in this leaflet, consult your doctor.
All medicines have benefits and risks. Your doctor has weighed the benefits that Evogam® will have for you against the possible risks.
If you have any concerns about using this medicine, ask your doctor. Follow your doctor's advice even if it is different from what this leaflet says.
Keep this leaflet with the medicine. You may need to read it again.
What Evogam® is used for
Your medicine is Evogam®, a solution for subcutaneous infusion. Evogam® contains human immunoglobulins and is manufactured from human plasma (the liquid component of blood) collected by Australian Red Cross Lifeblood.
Immunoglobulins are also called antibodies and are a type of protein found in the blood. Immunoglobulins are produced by your body’s immune system to fight infections caused by bacteria and viruses. If you do not have enough antibodies you may not be able to fight off diseases. Evogam® can be used as antibody replacement therapy, to correct this lack of antibodies.
Your doctor may have prescribed Evogam® for another reason. Ask your doctor if you have any questions about why it has been prescribed for you.
Before you receive Evogam®
When you must not have it
Do not have Evogam® if you are allergic to:
- human immunoglobulin products
- glycine.
If you are not sure whether you should be given this medicine talk to your doctor.
Before you are given it
Tell your doctor if you:
- are pregnant or breast-feeding
- have had any vaccination within the last two weeks
- are allergic to any medicine or food
- have IgA deficiency
- have a history of heart, or blood vessel disease, or blood clots, have thick blood, have been immobile for some time. Also tell the doctor what medicine you are using as some medicines, such as those that contain the hormone estrogen (for example, birth control pills), may increase your risk of developing a blood clot.
- have blood group A, B or AB
- have any other medical conditions.
If you have not told your doctor about any of the above, tell them before you are given Evogam®.
Your doctor can discuss with you the risks and benefits involved with using this medicine.
About blood products
When medicines are made from human blood or plasma, processes are used to prevent infections being passed from the blood/plasma donor to the person receiving the medicine. These processes include careful selection of the people who donate blood and plasma to make sure that those who might be carrying infections are excluded. In addition each donation and pools of donations are tested for indicators of virus or virus infection(s).
Manufacturers of these medicines also include steps in the processing of blood or plasma that inactivate or remove viruses. Despite these processes, when medicines are prepared from human blood or plasma, the possibility of passing on an infection cannot be totally ruled out. Unknown or new viruses or other types of infection could also be passed on.
The measures taken in the manufacture of this medicine are considered effective for enveloped viruses such as human immunodeficiency virus (HIV), hepatitis B virus, and hepatitis C virus, and for the non-enveloped viruses hepatitis A and B19 virus (B19V).
There is reassuring clinical experience regarding the lack of hepatitis A or B19V infections following treatment with immunoglobulin products. The antibodies which are in Evogam® may also make an important contribution to limiting the possibility an infection could be passed on.
Please discuss the risks and benefits of this product with your doctor.
Taking other medicines
Tell your doctor if you are taking any other medicines, including medicines that you buy without a prescription from your pharmacy, supermarket or health food shop.
Some medicines may affect the way other medicines work.
How Evogam® is given
Treatment should be started and supervised by a doctor. Evogam® is administered as an injection or infusion subcutaneously (under the skin). If your doctor decides that you should receive Evogam® at home, they will ensure you receive detailed instructions and training on how to use it.
If you do not understand the instructions ask your doctor or health professional.
How much is given
Your doctor will determine the dose(s) of Evogam® that you will receive, and when you need to have it.
How to prepare it
If your doctor considers that you should receive Evogam® at home, the instructions below should be followed carefully.
Step 1
Prepare Evogam® and equipment
a. Remove from the refrigerator and allow the vial of Evogam® to reach room temperature prior to use, this will take approximately 20-60 minutes.
b. Gather what you need for administration. This may include an infusion pump, administration tubing, subcutaneous needle or catheter set, Y-site connector, alcohol wipes, syringes, vial adapter, gauze or transparent dressing, tape, sharps disposal container, and treatment diary.
c. Check Evogam® vials. Carefully check the liquid in each vial, but do not shake it. Evogam® is clear and colourless or pale-yellow or light-brown. If Evogam® appears to be turbid or to contain sediment, it must not be used. If it is missing the protective cap, or is past its expiry date, it must not be used.
d. Remove the protective cap from the vial and wipe the rubber stopper with an alcohol wipe.
Step 2
Wash hands with soap and water and dry hands thoroughly with a clean towel.
Step 3
Prepare vial adapter
a. Remove the top cover of the vial adapter, leaving the adapter inside the blister pack.
b. Place the Evogam® vial on a flat surface.
c. Place the vial adapter over the top of the vial, using the blister pack to handle the vial adapter. Press down firmly until the vial adapter snaps into place so that it pierces the rubber stopper (see Figure 1).

d. Remove the outer package of the vial adapter and discard. Take care not to touch the exposed end of the device.
Step 4
Prepare syringe
Pull back the plunger of the syringe to fill the syringe with air. The amount of air should be the same as the amount of Evogam® you will transfer from the vial (see Figure 2).
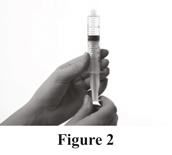
Step 5
Connect to vial
a. Attach the syringe to the vial adapter by twisting the syringe onto the connection.
b. Push the plunger of the syringe down. This will inject the air from the syringe into the airspace of the vial (see Figure 3).

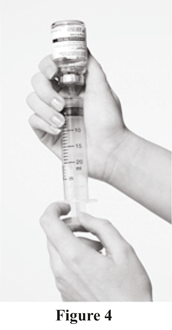
Step 6
Withdraw Evogam® from vial
a. Turn the vial and syringe upside down whilst holding the plunger in, leaving the syringe and vial attached (see Figure 4).
b. Release pressure on the plunger and pull back slowly to fill the syringe with Evogam®.
c. Detach the syringe from the vial adapter with a twist, leaving the vial adapter attached to the vial (see Figure 5).
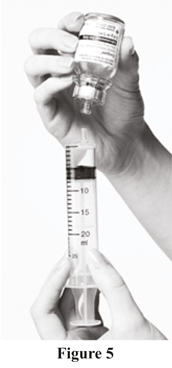
Repeat Steps 3 to 6 if multiple vials are required to achieve the prescribed dose of Evogam®. The same syringe may be used (to a maximum of 20 mL) but use a new vial adapter for each vial.
Step 7
Prepare infusion tubing
To prime (fill) the tubing, connect the syringe filled with Evogam® to the infusion tubing and gently push on the syringe plunger to fill the tubing with Evogam® (see Figure 6).
Stop priming before Evogam® fluid reaches the needle.

Step 8
Insert needle
a. Select an appropriate infusion site. This may be the abdomen, thigh, upper arm, or side of the hip.
The number and location of injection sites depends on the volume of the total dose. When a dose of more than 20 mL is given, it is advisable to give it in divided doses at different sites, which can be infused at the same time.
b. Clean the injection site(s) as directed by your health care professional.
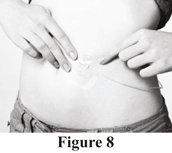
c. Pinch together the skin around the injection site with two fingers and insert the needle under the skin (see Figure 7).
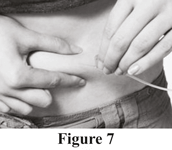
d. Place sterile gauze and tape or a transparent dressing over the injection site. This will keep the needle from coming out (see Figure 8).
Step 9
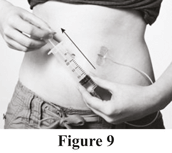
Check needle placement
Make sure you are not injecting Evogam® into a blood vessel. To test for this pull the plunger back gently (see Figure 9). If there is blood in the tubing, which would appear as red or pink fluid entering the tubing from the injection site, remove and discard the needle and tubing. Repeat steps beginning with Step 7 using a new needle and tubing, and a new infusion site.
If there is no blood in the tubing, continue to Step 10.
Step 10
Start infusion
Follow the instructions that you have been given by your doctor or nurse to start the infusion, and infuse at the rate you have been instructed.
The recommended initial infusion rate is 10 mL/hr per injection site, with subsequent infusion rate increased as tolerated to a maximum of 20 mL/hr per site.
Step 11
After the infusion
a. Take off the dressing and take the needle out of the injection site, applying light pressure to the injection site.
b. Throw away the Evogam® single-use vial, along with the needle and tubing, in the sharps container. Throw away dressing and tapes in the household waste.
c. Record your treatment. Peel off the removable part of the label of the Evogam® vial and put this label in your treatment diary or logbook. Document the date and time of your infusion, the amount of Evogam® that you infused and the rate of infusion.
If too much is given (overdose)
The effects of an overdose of Evogam® are not known. Please tell your doctor if you accidently use more than instructed.
Things you must do
Tell your doctor if you are planning on having a vaccination.
Evogam® may impair the effect of some virus vaccines such as measles, mumps, rubella and chicken pox for a period of at least six weeks, and up to three months. After receiving this medicine, a period of three months should be allowed before vaccination with some virus vaccines. In the case of measles vaccine, this effect may last for up to one year. Therefore, your vaccinating doctor should check the effectiveness of the measles vaccination.
Tell your doctor if you are about to have any blood tests.
Evogam® may interfere with the results of some tests, resulting in misleading results for some things.
Inform other doctors, dentists, and pharmacists who treat you that you have been given this medicine.
It is important for them to know if they are starting you on any other new medicines.
Side effects
All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical treatment if you experience some of the side effects.
Do not be alarmed by the following lists of possible side effects. You may not experience any of them. If you have any questions, ask your doctor.
Tell your doctor immediately or go to the Accident and Emergency Department at your nearest hospital if you notice any of the following symptoms:
- severe headache
- neck stiffness
- inability to stand bright light
- painful eye movements
- tingling, numbness or weakness on one side of the body
- pain/tenderness, swelling/discolouration of an arm or leg
- shortness of breath
- chest pain
- allergic or anaphylactic reaction, symptoms of which may include:
- swelling of the lips, tongue or eyes
- loss of consciousness
- hives
- difficulty in breathing - feeling very tired
- skin becoming yellow
- dark urine.
Tell your doctor if you notice any of the following and they worry you:
This list includes the more common side effects of Evogam®. They are usually mild and often reduce over time:
- pain, itching, redness or swelling where the injection was given
- nausea or vomiting
- back and muscle pain
- abdominal pain
- paleness of skin
- diarrhoea
- headache
- fever or chills
- feeling faint (fall in blood pressure)
- pain in extremity
- fatigue
- joint pain.
Other side effects not listed above may also occur in some patients. Tell your doctor if you notice any other effects.
After having Evogam®
Storage
Store at 2°C to 8°C. (Refrigerate. Do not freeze).
Once removed from refrigeration, store below 25°C and use within 2 weeks.
The product must not be returned to refrigeration.
Leave vial in box until ready to use.
Do not use after the expiry date.
Keep it out of the reach and sight of children.
Product description
What it looks like
Evogam® is clear and colourless or pale-yellow or light-brown, and may be turbid in appearance. It is packaged in single use clear glass vials.
Ingredients
Each vial of Evogam® contains a sterile solution comprising 16% plasma proteins of which at least 98% are immunoglobulins. Evogam® does not contain any preservatives, so any unused portion should be discarded immediately. Evogam® is packaged using latex free materials.
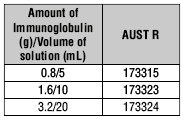
Evogam® is available in three different sizes, as shown in the table:
Manufacturer
Evogam® is manufactured in Australia by:
CSL Behring (Australia) Pty Ltd
ABN 48 160 734 761
189-209 Camp Road
Broadmeadows VIC 3047
Australia
Distributor
Australian Red Cross Lifeblood
Date of revision
August 2021
® Registered trademark of CSL Limited
Published by MIMS September 2021
