What is in this leaflet
This leaflet answers some common questions about FINPRO. It does not contain all the available information.
It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking FINPRO against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine. You may need to read it again.
What FINPRO is used for
FINPRO is for use by men only.
FINPRO is used to treat a medical condition in men called benign prostatic hyperplasia or BPH. BPH is a condition where your prostate gland (which is near your bladder) has become bigger making it more difficult for you to pass urine. This can lead to symptoms such as:
- weak or interrupted stream of urine
- feeling that you cannot empty your bladder completely
- delay before you start to pass urine
- needing to pass urine often, especially at night
- feeling that you must pass urine right away
BPH occurs only in men and is common over the age of 50 years. In some men, BPH can lead to serious problems, including urinary tract infections and the sudden inability to pass urine at all. BPH can also lead to the need for surgery such as procedures to improve the flow of urine.
The prostate gland takes years to grow. Therefore, the symptoms of BPH take a long time to develop. FINPRO works by slowly reducing the size of your prostate gland. This may lead to gradual improvement in your urine flow and other symptoms over several months. FINPRO also helps reduce the risk of developing a sudden inability to pass urine (acute urinary retention) and the need for surgery. This may happen whether or not you notice any improvement or change in your symptoms.
Your doctor may have prescribed FINPRO for another reason. Ask your doctor if you have any questions about why FINPRO has been prescribed for you.
FINPRO is not addictive.
Before you take FINPRO
When you must not take it
Do not take FINPRO if:
-
you have an allergy to FINPRO or any of the ingredients listed at the end of this leaflet
Symptoms of an allergic reaction to FINPRO may include skin rash, or swelling of the lips or face. - the packaging is torn or shows signs of tampering
-
the expiry date on the pack has passed.
If you take this medicine after the expiry date has passed, it may not work.
If you are not sure whether you should start taking FINPRO, talk to your doctor.
Do not give FINPRO to children or women.
Women who are pregnant or may be pregnant must not take FINPRO or handle crushed or broken tablets. If the active ingredient in FINPRO is absorbed after swallowing the tablet or through the skin by a woman who is pregnant with a male baby, it may cause the male baby to be born with abnormalities of the sex organs. FINPRO tablets are coated and will prevent contact with the active ingredient during normal handling, provided the tablets are not broken or crushed.
If a pregnant woman swallows FINPRO or handles crushed or broken tablets, her doctor must be consulted immediately.
The condition for which FINPRO is prescribed occurs only in men.
Before you start to take it
Tell your doctor if:
- you have or have had any medical conditions
- you have any allergies to any other medicines or any other substances, such as foods, preservatives or dyes.
If you have not told your doctor about any of the above, tell them before you take any FINPRO.
Taking other medicines
Tell your doctor if you are taking any other medicines, including medicines that you buy without a prescription from your pharmacy, supermarket or health food shop.
Some medicines may affect the way other medicines work. However, FINPRO has not been shown to interfere with other medicines.
Driving and operating machinery
FINPRO should not affect your ability to drive or operate machinery.
How to take FINPRO
How much to take
Take FINPRO only when prescribed by your doctor. The usual dose in men is one tablet taken once each day.
Swallow FINPRO with a glass of water.
Follow all directions given to you by your doctor carefully. They may differ from the information contained in this leaflet.
If you do not understand the instructions on the box, ask your doctor or pharmacist for help.
When to take it
Take FINPRO once a day at about the same time each day. This will help you to remember to take the tablets.
It does not matter if you take FINPRO before or after food.
How long to take it
FINPRO shrinks the prostate gland slowly. Therefore, you may need to take FINPRO for 6 months or longer to see whether it helps you. If it does help your symptoms, you may need to take FINPRO every day. Continue taking FINPRO for as long as your doctor prescribes. If you stop taking the medicine the prostate gland is likely to grow again.
If you forget to take it
If it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to. Otherwise, take it as soon as you remember, and then go back to taking your tablet as you would normally.
If you are not sure whether to skip the dose, talk to your doctor or pharmacist.
Do not take a double dose to make up for the dose that you missed.
If you have trouble remembering to take your tablets, ask your pharmacist for some hints.
If you take too much (overdose)
Immediately telephone your doctor or Poisons Information Centre (telephone 13 11 26) for advice, if you think that you or anyone else may have taken too much FINPRO. Do this even if there are no signs of discomfort or poisoning.
While you are using FINPRO
Things you must do
Go to your doctor for regular checkups, including a physical check for prostate cancer once a year if you are over 50.
While BPH is not cancer and does not lead to cancer, the two conditions can exist at the same time.
FINPRO is used for BPH not prostate cancer.
If you are having a blood test to measure your PSA (prostate-specific antigen) levels, tell your doctor you are taking FINPRO. FINPRO can affect the results of this test.
If you are about to be started on any new medicine tell your doctor and pharmacist that you are taking FINPRO.
Things you must not do
Do not give FINPRO to anyone else, even if they have the same condition as you.
Side Effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking FINPRO.
FINPRO helps most men with BPH, but it may have unwanted side effects in a few people. All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical treatment if you get some of the side effects.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor if you notice any of the following and they worry you:
- impotence (inability to have an erection) that continues after stopping FINPRO
- less desire for sex that continues after stopping FINPRO
- problems with ejaculation that continued after stopping the medication
Changes or problems with ejaculation, such as decreased amount of semen released during sex (this decrease does not appear to interfere with normal sexual function).
Male infertility and/or poor quality of semen have been reported infrequently. Improvement in the quality of semen has been reported after stopping FINPRO.
These are the more common side effects of FINPRO. For the most part these have been mild. In some cases, these side effects disappeared while the patient continued to take FINPRO. If symptoms persisted, they usually resolved on stopping the tablets.
Tell your doctor immediately if you notice any of the following:
- breast swelling and/or tenderness
In rare cases, male breast cancer has been reported. - breast lumps, pain or discharge from the nipples.
- skin rash, itchiness
- hives or nettlerash (pinkish, itchy swellings on the skin)
- testicle pain
- blood in semen
- depressions (feelings of severe sadness and unworthiness) including suicidal thoughts
These are uncommon side effects that have been reported with FINPRO.
Tell your doctor immediately or go to accident and emergency at your nearest hospital if the following happens:
- swelling of the lips, tongue, throat or face
These may be symptoms of a serious allergic reaction to FINPRO, which may cause difficulty in swallowing or breathing. You may need urgent medical attention. Serious side effects are rare.
Other side effects not listed above may also occur in some patients. Tell your doctor if you notice any other effects.
Do not be alarmed by this list of possible side effects. You may not experience any of them.
After using FINPRO
Storage
Keep your tablets in the blister pack until it is time to take them. If you take the tablets out of the blister pack they may not keep well.
Never put the tablets in another box or container, as they might get mixed up.
Keep FINPRO in a cool dry place away from light where the temperature stays below 30°C. Do not store it or any other medicine in the bathroom or near a sink.
Do not leave it in the car or on window sills. Heat and dampness can destroy some medicines.
Keep it where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking the tablets, or the tablets have passed their expiry date, ask your pharmacist what to do with any that are left over.
Product description
What it looks like
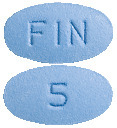
FINPRO comes as a blue coloured, oval shaped, biconvex, film-coated tablet, debossed with 'FIN' on one side and '5' on the other side.
A pack contains 30 tablets in a blister pack.
Ingredients
Active ingredient:
- finasteride 5 mg per tablet
Inactive ingredients:
- lactose monohydrate
- pregelatinised maize (corn) starch
- sodium starch glycollate
- docusate sodium
- microcrystalline cellulose
- magnesium stearate
- Opadry complete film-coating system 03B50899 blue.
FINPRO does not contain gluten, sucrose, tartrazine or any other azo dyes.
Supplier
Dr Reddy’s (Australia) Pty Ltd
Melbourne, VIC, 3004
AUSTRALIA
This leaflet was prepared in March 2020.
Australian Register Number: AUST R 161627
Published by MIMS May 2020