What is in this leaflet
This leaflet answers some common questions about Galvumet.
It does not contain all the available information. It does not take the place of talking to your doctor, pharmacist or diabetes educator.
The information in this leaflet was last updated on the date listed on the final page. More recent information on the medicine may be available.
You should ensure that you speak to your pharmacist or doctor to obtain the most up to date information on the medicine. You can also download the most up to date leaflet from www.novartis.com.au. Those updates may contain important information about the medicine and its use of which you should be aware.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking this medicine against the benefits they expect it will provide.
If you have any concerns about this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine. You may need to read it again.
What Galvumet is used for
Galvumet is used to treat type 2 diabetes mellitus in people who are already taking vildagliptin and metformin tablets separately, or whose diabetes cannot be controlled by metformin alone.
Galvumet is also used with a sulfonylurea by patients whose blood sugar levels are not adequately controlled when taking only metformin and a sulfonylurea.
Galvumet is also added to insulin in patients when a stable dose of insulin and metformin do not provide adequate blood sugar control.
It is prescribed by your doctor together with diet and exercise.
Galvumet contains two ingredients: vildagliptin, which belongs to a class of medicines called 'islet enhancers', and metformin, which belongs to the 'biguanide' class.
Type 2 diabetes mellitus used to be known as 'non-insulin-dependent diabetes mellitus (NIDDM)' or 'maturity onset diabetes'.
Type 2 diabetes develops if the body does not produce enough insulin, or where the insulin that your body makes does not work as well as it should. It can also develop if the body produces too much glucagon.
Insulin is a substance which helps to lower the level of sugar in your blood, especially after meals. Glucagon is another substance which triggers the production of sugar by the liver, causing the blood sugar to rise. The pancreas makes both of these substances.
Galvumet helps to control the blood sugar level. It works by making the pancreas produce insulin and less glucagon (effect of vildagliptin) and also by helping the body to make better use of the insulin it produces (effect of metformin).
Your doctor will prescribe Galvumet either alone or in combination with another antidiabetic medicine to replace the antidiabetic medicine(s) you are already taking, where that medicine(s) alone is not enough to control your blood sugar level.
It is important that you continue to follow the diet and/or exercise recommended for you whilst you are on treatment with Galvumet.
Ask your doctor if you have any questions about why this medicine has been prescribed for you. Your doctor may have prescribed it for another reason.
Galvumet is not a substitute for insulin. It is not used to treat type 1 diabetes (where your body does not produce insulin at all), or diabetic ketoacidosis.
This medicine is only available with a doctor's prescription. It is not addictive.
There is not enough information to recommend this medicine for use in children under 18 years old.
Before you take Galvumet
When you must not take it
Do not take this medicine if you have an allergy to:
- vildagliptin or metformin (the active ingredients) or to any of the other ingredients listed at the end of this leaflet.
- any other similar medicines (such as medicines of the same class or with a similar structure, if listed in the PI)
Some of the symptoms of an allergic reaction may include shortness of breath; wheezing or difficulty breathing; swelling of the face, lips, tongue or other parts of the body; rash, itching or hives on the skin.
Do not take this medicine if you have any of the following:
- severely reduced kidney function where your doctor has considered use of Galvumet to be unsuitable
- taken this medicine before and your doctor told you to stop taking it because of liver problems
- had a recent heart attack or have heart failure
- serious circulation problems, including shock and breathing difficulties
-
serious complications of your diabetes
for example diabetic ketoacidosis (a complication of diabetes involving rapid weight loss, nausea or vomiting) or diabetic coma -
type 1 diabetes
(a condition where your body does not produce any insulin at all. Galvumet is not a substitute for insulin) -
diabetic ketoacidosis
(a complication in patients with diabetes mellitus who have little to no insulin. Galvumet is not a substitute for insulin)
Do not take this medicine if you are going to have a contrast x-ray (a type of x-ray involving an injectable dye). This medicine may affect your kidney function so you will need to stop taking it before or at the time of the procedure and for a few days after.
Do not take this medicine after the expiry date printed on the pack or if the packaging is torn or shows signs of tampering. In that case, return it to your pharmacist.
Before you start to take it
Discard any other medicines containing metformin that your doctor might have prescribed to you in the past and that you may still have in your possession. Galvumet contains metformin. If you have more than one metformin-containing medicine in your possession you may accidentally take too much (overdose). Accidentally taking too much metformin can cause a very serious side effect called lactic acidosis.
ACCIDENTAL METFORMIN OVERDOSING IS A SIGNIFICANT SAFETY RISK.
Ask your doctor or pharmacist if you are unsure if you have any other medicines containing metformin. Metformin is sold under many different brand names in Australia. Your doctor or pharmacist will know which other medicines also contain metformin.
Tell your doctor if you have allergies to any other medicines, foods, dyes or preservatives. Your doctor will want to know if you are prone to allergies.
Tell your doctor if you are pregnant or intend to become pregnant. Your doctor will discuss the possible risks and benefits involved.
Tell your doctor if you are breast-feeding or plan to breast-feed. It is not known if the active ingredient of Galvumet passes into breast milk and could affect your baby.
Tell your doctor if you have any of the following medical conditions:
- problems with your kidneys
- problems with your liver
- type 1 diabetes (formerly called 'juvenile onset' or 'insulin-dependent' diabetes mellitus or 'IDDM'), where the body does not produce any insulin
- diabetic ketoacidosis, a condition where chemicals called ketones build up in the body due to very low insulin levels
If you are not sure whether any of the above conditions apply to you, your doctor can advise you.
Your doctor will do some blood and urine tests for sugar level regularly, and for liver and kidney function at the start of treatment and regularly while you are on treatment.
Alcohol, diet, exercise and your general health all strongly affect the control of your diabetes.
Taking other medicines
Tell your doctor or pharmacist if you are taking any other medicines, including any that you buy without a prescription from a pharmacy, supermarket or health food shop.
You may need to take different amounts of your medicines or to take different medicines while you are taking Galvumet. Your doctor and pharmacist have more information.
This is particularly important with the following medicines:
- Certain medicines used to treat infections (e.g. vancomycin, trimethoprim)
- Certain medicines used to treat inflammation (e.g. corticosteroids)
- Certain medicines used to treat high blood pressure (e.g. amiloride, triamterene, nifedipine, enalapril, losartan, diuretics)
- Certain medicines used to treat irregular heartbeat (e.g. digoxin, quinidine)
- Certain medicines used to treat pain (e.g. morphine, diclofenac)
- Certain medicines used to treat stomach disorders (e.g. cimetidine, ranitidine)
- Certain medicines used to treat psychiatric disorders (e.g. phenothiazine)
- Certain medicines used to treat thyroid disorders
- Oral contraceptives and certain medicines used to reduce symptoms in women experiencing menopause or osteoporosis (e.g. oestrogen)
- If you need to have an injection of a contrast medium that contains iodine into your bloodstream, for example in the context of an X-ray or scan, you must stop taking Galvumet before or at the time of the injection. Your doctor will decide when you must stop and when to restart your treatment with Galvumet.
If you have not told your doctor about any of these things, tell him/her before you start taking this medicine.
How to take Galvumet
Follow all directions given to you by your doctor and pharmacist carefully. They may differ from the information contained in this leaflet.
If you do not understand the instructions on the label, ask your doctor or pharmacist for help.
How much to take
The usual dose of Galvumet is one tablet twice a day. Your doctor will tell you exactly how many tablets to take. Do not exceed two tablets a day.
Your doctor will monitor your blood glucose levels and may increase or decrease the dose of Galvumet to maintain good control of your diabetes.
If you have reduced kidney function, your doctor may prescribe a lower dose. Also if you are taking an antidiabetic medicine known as a sulphonylurea your doctor may prescribe a lower dose.
How to take it
Swallow Galvumet tablets whole with a glass of water.
When to take it
Take your medicine at about the same time each day. Taking it at the same time each day will have the best effect. It will also help you remember when to take it.
Take this medicine either with or just after food. This will reduce the chance of you getting an upset stomach.
How long to take it
Continue taking your medicine for as long as your doctor tells you to. Your doctor will check your progress to make sure the medicine is working and will discuss with you how long your treatment should continue.
Do not stop taking Galvumet unless your doctor tells you to.
If you forget to take it
If it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to.
Otherwise, take it as soon as you remember, and then go back to taking your medicine as you would normally.
Do not take a double dose to make up for the dose that you missed. This may increase the chance of you getting an unwanted side effect.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering when to take your medicine, ask your pharmacist for some hints.
If you take too much (overdose)
Immediately telephone your doctor or Poisons Information Centre (telephone number: 13 11 26), or go to Accident and Emergency at your nearest hospital if you think that you or anyone else may have accidentally taken too much Galvumet. Do this even if there are no signs of discomfort or poisoning. Keep the telephone numbers for these places handy. You may need urgent medical attention.
Symptoms of an overdose may include:
- swelling in hands or feet
- tingling or numbness in hands or feet
- muscle pain
- fever
Symptoms of an overdose can also include the symptoms of lactic acidosis:
- feeling cold (especially in your arms and legs)
- feeling very weak, tired
- feeling light-headed, dizzy
- severe nausea or vomiting
- feeling uncomfortable
- muscle pain
- drowsiness
- abdominal pain
- unexplained weight loss
- irregular heartbeat
- rapid or difficult breathing
While you are taking Galvumet
Things you must do
If you become pregnant while taking this medicine, tell your doctor immediately. Galvumet should not be taken if you are pregnant. Insulin is more suitable for controlling blood glucose during pregnancy.
Carefully follow your doctor's and/or dietician's advice on diet, drinking alcohol and exercise.
Keep all of your doctor's appointments so that your progress can be checked.
Your doctor will do regular checks to help prevent you from having side effects from the medicine or developing serious complications of diabetes. These will include tests for:
- Blood and urine:
- These should be regularly tested for sugar - Kidney function:
- This should be checked at start of treatment and at least once a year whilst you are on treatment.
- This should be checked more often if you are elderly. - Liver function:
- This should be checked at start of treatment and every 3 months during your first year of treatment, and regularly thereafter. - General blood tests:
- These should be done at least once a year. - Vitamin B12 levels:
- This will be checked at least every 2 to 3 years.
Make sure you check your blood glucose levels regularly. This is the best way to tell if your diabetes is being controlled properly. Your doctor or diabetes educator will show you how and when to do this.
Tell your doctor if you become ill or experience extra stress, injury, fever, infection or need surgery. Your blood glucose may become difficult to control at these times.
Make sure you keep enough medicine to last over weekends and holidays. It is important to keep your blood glucose controlled at all times to prevent serious complications of diabetes from happening.
Remind your doctor and pharmacist that you are taking Galvumet if you are about to be started on any new medicine.
Tell any other doctor, dentist or pharmacist who treats you that you are taking Galvumet.
Things you must not do
Do not give this medicine to anyone else, even if their condition seems similar to yours.
Do not use it to treat any other complaints unless your doctor tells you to.
Things to be careful of
Be careful driving, operating machinery or doing jobs that require you to be alert until you know how this medicine affects you. If your blood glucose level becomes too low, you may feel dizzy, lightheaded, weak or tired and your reaction time may be slower than usual. If you have any of these symptoms, do not drive or do anything else that could be dangerous.
Be careful when doing any of the following things, which increase the risk of your blood glucose becoming too low:
- drinking alcohol
- not eating enough
- doing unexpected or vigorous exercise
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking Galvumet even if you do not think it is connected with the medicine.
All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical treatment if you get some of the side effects.
Do not be alarmed by these lists of possible side effects.
You may not experience any of them.
Ask your doctor, pharmacist or diabetes educator to answer any questions you may have.
Tell your doctor if you notice any of the following side effects and they worry you:
- skin reddening or itching
- peeling of skin or blisters
- skin lesions
- joint pain
- swelling of the hands, ankles or feet
- low blood glucose
- diarrhoea
- abdominal pain
- a burning sensation in the chest rising up to the throat ('heartburn')
- metallic taste
- loss of appetite
- constipation
- wind (flatulence)
- weight increase
Stop taking Galvumet and tell your doctor immediately or go to Accident and Emergency if you notice any of the following:
- swelling of the face, lips, mouth, tongue or throat which may cause difficulty in swallowing or breathing, sudden onset of rash or hives
These are symptoms of severe allergic reaction. - yellow skin and eyes, nausea, loss of appetite, dark urine (possible symptoms of liver problems)
These are symptoms of liver problems. - nausea, excessive sweating, weakness, dizziness, trembling, headache, chills
These are signs of a low blood sugar level, which could be due to lack of food, too much exercise without enough food or too much alcohol. - Severe stomach pain
This is a possible sign of an inflamed pancreas or gallbladder.
Stop taking Galvumet if you get any of the symptoms of lactic acidosis and go to Accident and Emergency immediately.
Metformin has caused lactic acidosis in rare cases. This is a medical emergency that can cause death. It is caused by build-up of lactic acid in your blood. The symptoms of lactic acidosis are:
- feeling cold (especially in your arms and legs)
- feeling very weak, tired
- feeling light-headed, dizzy
- severe nausea or vomiting
- feeling uncomfortable
- muscle pain
- drowsiness
- abdominal pain
- unexplained weight loss
- irregular heartbeat
- rapid or difficult breathing
Tell your doctor if you notice anything else that is making you feel unwell. Some people may have other side effects not yet known or mentioned in this leaflet. Some side effects (e.g. changes in liver function) can only be found by laboratory testing.
After using Galvumet
Storage
Keep your medicine in the original container until it is time to take it.
Store it in a cool dry place where the temperature stays below 30°C.
Protect from moisture.
Do not store Galvumet or any other medicine in the bathroom or near a sink.
Do not leave it in the car or on window sills.
Keep the medicine where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking this medicine or the expiry date has passed, ask your pharmacist what to do with any tablets you have left over.
Product description
What it looks like
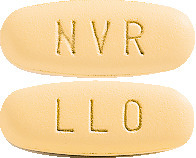
Galvumet is a yellow, oval tablet imprinted as follows:
- "NVR" on one side and "LLO" on the other side (50/500 tablet).
- "NVR" on one side and "SEH" on the other side (50/850 tablet).
- "NVR" on one side and "FLO" on the other side (50/1000 tablet).
Galvumet is available in blister packs containing 10, 30, 60, 120 or 180 tablets.
Some pack sizes may not be marketed.
Ingredients
Each tablet of Galvumet contains two active substances: vildagliptin and metformin hydrochloride. Three tablet strengths are available, each containing the following combinations of vildagliptin/metformin:
- 50 mg/500 mg
- 50 mg/850 mg
- 50 mg/1000 mg
Each tablet also contains the following inactive ingredients:
- iron oxide red
- iron oxide yellow
- hypromellose
- hydroxypropyl cellulose
- Macrogol
- magnesium stearate
- Talc
- Titanium dioxide
Galvumet does not contain lactose, gluten, tartrazine or any other azo dyes.
Sponsor
Galvumet is supplied in Australia by:
NOVARTIS Pharmaceuticals Australia Pty Limited
ABN 18 004 244 160
54 Waterloo Road
Macquarie Park NSW 2113
Telephone: 1 800 671 203
Web site: www.novartis.com.au
® = Registered Trademark
This leaflet was prepared in October 2023
Australian Registration Numbers:
Galvumet 50/500 tablets - AUST R 161216
Galvumet 50/850 tablets - AUST R 161217
Galvumet 50/1000 tablets - AUST R 161218
Internal Document Code:
(CMI gam311023c based on PI gam311023i)
Published by MIMS December 2023