What is in this leaflet
This leaflet answers some common questions about Amlodipine besylate.
It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking Amlodipine besylate against the benefits it is expected to have for you.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine. You may need to read it again.
What this medicine is used for
The name of your medicine GTA-AMLODIPINE tablets. It contains the active ingredient amlodipine besylate.
What it does
This is used to:
- lower high blood pressure
(hypertension).
There are usually no symptoms of hypertension. The only way of knowing that you have hypertension is to have your blood pressure checked on a regular basis. If high blood pressure is not treated it can lead to serious health problems. - treat angina pectoris.
Angina is a pain or uncomfortable feeling in the chest, often spreading to the arms or neck, and sometimes to the shoulders and back. The pain of angina is due to a shortage of oxygen to the heart.
This medicine is not for the relief of a sudden attack of angina. Your doctor will give you other medication to treat this.
How it works
This medicine belongs to a group of medicines called calcium channel blockers or calcium ion antagonists. They work by widening your blood vessels, making it easier for your heart to pump blood around the body and help increase the supply of blood and oxygen to your heart. Calcium channel blockers do not change the amount of calcium in your blood or bones.
Ask your doctor if you have any questions about why this medicine has been prescribed for you. Your doctor may have prescribed it for another reason.
This medicine is available only with a doctor's prescription.
Use in Children
There is not enough information to recommend the use of this medicine in children.
Before you take this medicine
When you must not take it
Do not take this medicine if you have an allergy to:
- this medicine, or any other medicine containing amlodipine
- other calcium channel blockers such as medicines with the active ingredient felodipine, nifedipine or lercanidipine.
Check with your doctor or pharmacist if you are unsure.
- any of the ingredients listed at the end of this leaflet.
Some of the symptoms of an allergic reaction may include:
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body
- rash, itching or hives on the skin.
Do not take this medicine after the expiry date printed on the pack or if the packaging is torn or shows signs of tampering. If it has expired or is damaged, return it to your pharmacist for disposal.
If you are not sure whether you should start taking this medicine, talk to your doctor.
Before you start to take it
Tell your doctor if you have allergies to any other medicines, foods, preservatives or dyes.
Tell your doctor if you have or have had any of the following medical conditions:
- heart problems, including heart failure
- liver problems.
Tell your doctor if you are pregnant or plan to become pregnant. This medicine may affect your developing baby if you take it during pregnancy. Your doctor can discuss with you the risks and benefits involved.
Do not breast-feed if you are taking this medicine. It is not known if the active ingredient in this medicine passes into breast milk or if your baby may be affected.
If you have not told your doctor about any of the above, tell him/ her before you start taking this medicine.
Taking other medicines
Tell your doctor or pharmacist if you are taking any other medicines, including:
- all prescription medicines
- all medicines, vitamins, herbal supplements or natural therapies you buy without a prescription from a pharmacy, supermarket, naturopath or health food shop.
Some medicines may be affected by this medicine or may affect how well it works. You may need different amounts of your medicines, or you may need to take different medicines. Your doctor will advise you.
Tell your doctor or pharmacist if you are taking any of the following:
- other medicines used to treat angina, such as diltiazem
- some antibiotics, such as erythromycin, clarithromycin or rifampicin
- some antifungals, such as ketoconazole or itraconazole
- anti-proteases, medicines used to treat HIV infection, such as ritonavir
- simvastatin, a medicine used to lower cholesterol
- cyclosporin or tacrolimus, medicines used to suppress the immune system
- St John's Wort.
Your doctor and pharmacist have more information on medicines to be careful with or avoid while taking this medicine.
How to take this medicine
Take this medicine exactly as your doctor has prescribed.
Follow all directions given to you by your doctor or pharmacist carefully. They may differ from the information contained in this leaflet.
If you do not understand the instructions on the box, ask your doctor or pharmacist for help.
How much to take
The usual dose of this medicine is 5 mg each day. Your doctor may increase this to 10 mg each day.
Your doctor may prescribe another dose of this medicine depending on your condition and how you respond to this medicine.
How to take it
Swallow the tablet whole with a full glass of water.
When to take it
Take your medicine at about the same time each day, either morning or evening. Taking it at the same time each day will have the best effect. It will also help you remember when to take it.
this medicine can be taken with or without food.
How long to take it
You must take this every day. Continue taking your medicine for as long as your doctor tells you.
This medicine helps to control your condition, but does not cure it. It is important to keep taking your medicine even if you feel well.
If you forget to take it
If it is less than 12 hours before your next dose, skip the dose you missed and take your next dose when you are meant to.
Otherwise, take it as soon as you remember, and then go back to taking your medicine as you would normally.
Do not take a double dose to make up for the dose that you missed. This may increase the chance of you getting an unwanted side effect.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering to take your medicine, ask your pharmacist for some hints.
If you take too much (overdose)
Immediately telephone your doctor or Poisons Information Centre - the telephone number in Australia is 131 126 -for advice or go to Accident and Emergency at your nearest hospital if you think that you or anyone else may have taken too much this medicine.
Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
If you take too many tablets, you may feel dizzy, lightheaded or faint and have an irregular heartbeat.
While you are taking this medicine
Things you must do
Tell any other doctors, dentists and pharmacists who are treating you that you are taking this medicine.
If you are about to start any new medicine, tell your doctor or pharmacist that you are taking this medicine.
If you become pregnant while taking this medicine, tell your doctor immediately.
Keep all of your doctor's appointments so that your progress can be checked. Your doctor may do some tests from time to time to make sure the medicine is working and to prevent unwanted side effects.
Things you must not do
Do not take this medicine to treat any other conditions unless your doctor tells you to.
Do not give this medicine to anyone else, even if they have the same condition as you.
Things to be careful of
Avoid eating large quantities of grapefruit or drinking large quantities of grapefruit juice. Grapefruit juice contains one or more components that alter the metabolism of some medicines, including this medicine.
Drinking very large quantities (over 1.2 litres) of grapefruit juice each day while taking this medicine may increase the effects of this medicine.
Be careful driving or operating machinery until you know how this medicine affects you.
this medicine may cause dizziness or drowsiness in some people.
If you have any of these symptoms, do not drive, operate machinery or do anything else that could be dangerous.
Things that would be helpful for your high blood pressure or angina
Some self-help measures suggested below may assist your condition. Your doctor or pharmacist can give you more information about these measures.
- Weight: Your doctor may suggest losing some weight. Some people may need a dietician to plan a suitable diet to help with weight loss.
- Exercise: Regular exercise helps lower blood pressure and strengthen the heart. It is important not to overdo it. Before commencing regular exercise you should consult your doctor who will suggest the most suitable exercise for you. If you feel uncomfortable when exercising or experience symptoms such as chest pain or breathlessness see your doctor.
- Alcohol: Your doctor may advise you to limit your alcohol intake.
- Salt: Your doctor may advise you to watch the amount of salt in your diet. To reduce your salt intake you should avoid using salt at the table or in cooking.
- Smoking: Your doctor may advise you to stop smoking or at least cut down.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking this medicine.
this medicine helps most people but it may have some unwanted side effects in a few people. All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical attention if you get some of the side effects.
It can be difficult to tell whether side effects are the result of taking this medicine, effects of your condition or side effects of other medicines you may be taking. For this reason it is important to tell your doctor of any change in your condition.
Do not be alarmed by the list of possible side effects. You may not experience any of them.
Tell your doctor if...
Ask your doctor or pharmacist for advice if you experience any of the following and they worry you:
- headache
- dizziness
- flushing
- tiredness
- drowsiness or sleepiness
- stomach pain or nausea.
These are the more common side effects of this medicine. All side effects should be reported to a health professional.
Tell your doctor or pharmacist if you experience any of the following and they worry you:
- indigestion
- sexual problems.
These may or may not be due to this medicine but you should tell your doctor.
Tell your doctor as soon as possible if...
Tell your doctor immediately if you notice any of the following:
- changes in heart beat either fast or slow
- swelling of the ankles, feet, face or hands
- tingling or numbness of the hands or feet
- dizziness or lightheadedness on standing up from a sitting or lying position
- unusual tiredness or weakness
- muscle cramps or aches
- joint pain
- eye pain or change in vision
- changes in mood, feeling anxious or nervous
- symptoms of liver disease such as itching, yellowing of the skin and eyes, and dark coloured urine
- unusual movements, including trembling and shaking of the hands and fingers, twisting movements of the body, shuffling walk and stiffness of the arms and legs.
These may be serious side effects that may need urgent medical attention
Go to hospital if...
Tell your doctor immediately or go to Accident and Emergency at your nearest hospital, if you notice any of the following:
- fast or irregular heart beats
- chest pain
- chest pain associated with exertion (angina) that lasts longer, is more severe or occurs more often
- shortness of breath
- symptoms of allergy such as skin rash and/or itching
- severe upper stomach pain, often with nausea and vomiting.
The above list includes very serious side effects. You may need urgent medical attention or hospitalisation.
Ask your doctor or pharmacist to answer any questions you may have.
If you are 65 years or older, you should be especially careful while taking this medicine. Report any side effects promptly to your doctor. Some people in this age group may be more likely to experience side effects such as swelling of the feet and ankles, muscle cramps and dizziness.
Tell your doctor or pharmacist if you notice anything else that is making you feel unwell. Other side effects not listed above may also occur in some people.
After taking this medicine
Storage
Keep your tablets in the pack until it is time to take them. If you take your tablets out of the pack they may not keep as well.
Keep your tablets in a cool dry place where the temperature stays below 25°C.
Do not store this medicine or any other medicine in the bathroom or near a sink. Do not leave your medicines on a window sill or in the car. Heat and dampness can destroy some medicines.
Keep this medicine where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking this medicine, or the tablets have passed their expiry date, ask your pharmacist what to do with any medicine that is left over.
Product description
What it looks like
This medicine tablets are available in two strengths:
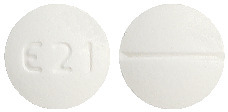
- 5 mg tablets – white to off white, circular, biconvex, uncoated tablets debossed with ‘E21’ on one side and score line on the other side.
- 10 mg tablets - white to off white, round, uncoated tablets with ‘10’ debossing on one side.
The 5 mg and 10 mg tablets come in packs of 10's and 14’s. Further blisters are packed into an outer carton to give pack sizes of 10, 28, 30, 50, 56 and 84 tablets.
Ingredients
Active ingredients
- 5 mg tablets contain amlodipine besylate equivalent to amlodipine 5 mg.
- 10 mg tablets contain amlodipine besylate equivalent to amlodipine 10 mg.
Other ingredients
- microcrystalline cellulose
- calcium hydrogen phosphate
- sodium starch glycollate
- Silica-colloidal anhydrous
- magnesium stearate.
This medicine does not contain gluten, sugar or lactose.
Supplier
Ipca Pharma (Australia) Pty Ltd,
6 Morotai Avenue Ashburton VIC 3147, Australia
This leaflet was prepared in
February 2021
Australian Registration Number:
5 mg -264394 and 10 mg-264395
Published by MIMS October 2021