Hadlima® PushTouch® Auto-injector 40 mg
| Consumer Medicine Information (CMI) summary |
The full CMI on the next page has more details. If you are worried about using this medicine, speak to your doctor or pharmacist.
| 1. Why am I using Hadlima? |
Hadlima contains the active ingredient adalimumab. Hadlima is used to treat various inflammatory conditions.
For more information, see Section 1. Why am I using Hadlima? in the full CMI.
| 2. What should I know before I use Hadlima? |
Do not use if you have ever had an allergic reaction to Hadlima or any of the ingredients listed at the end of the CMI.
Talk to your doctor if you have any other medical conditions, take any other medicines, or are pregnant or plan to become pregnant or are breastfeeding.
For more information, see Section 2. What should I know before I use Hadlima? in the full CMI.
| 3. What if I am taking other medicines? |
Some medicines may interfere with Hadlima and affect how it works, or Hadlima may interfere with other medicines and affect how they work.
A list of these medicines is in Section 3. What if I am taking other medicines? in the full CMI.
| 4. How do I use Hadlima? |
Hadlima is injected under the skin (subcutaneous).
More instructions can be found in Section 4. How do I use Hadlima? in the full CMI.
| 5. What should I know while using Hadlima? |
| Things you should do |
|
| Things you should not do |
|
| Driving or using machines |
|
| Drinking alcohol |
|
| Looking after your medicine |
|
For more information, see Section 5. What should I know while using Hadlima? in the full CMI.
| 6. Are there any side effects? |
Side effects that require urgent medical attention include: Signs of an allergic reaction, such as chest tightness, difficult y breathing, swelling of face lips and tongue, rash; signs of heart failure, such as shortness of breath on exertion or lying down, swelling of the feet; signs suggesting a blood disorder, such as persistent fever, bruising, bleeding, paleness.
For more information, including what to do if you have any side effects, see Section 6. Are there any side effects? in the full CMI.
Hadlima® PushTouch® Auto-injector 40 mg
Active ingredient(s): adalimumab (rch) (a-da-li-mue-mab)
| Consumer Medicine Information (CMI) |
This leaflet provides important information about using Hadlima. You should also speak to your doctor or pharmacist if you would like further information or if you have any concerns or questions about using Hadlima.
Where to find information in this leaflet:
1. Why am I using Hadlima?
2. What should I know before I use Hadlima?
3. What if I am taking other medicines?
4. How do I use Hadlima?
5. What should I know while using Hadlima?
6. Are there any side effects?
7. Product details
| 1. Why am I using Hadlima? |
Hadlima contains the active ingredient adalimumab.
Hadlima is used for the treatment of a number of inflammatory diseases:
Rheumatoid arthritis
Rheumatoid arthritis is an inflammatory disease of the joints. Signs and symptoms of rheumatoid arthritis include joint pain, tenderness, swelling and stiffness.
Hadlima is used to reduce the signs and symptoms of moderate to severely active rheumatoid arthritis, as well as to slow down and protect the joints from further damage to help them move more freely.
Your doctor will decide if Hadlima should be used with another medicine called methotrexate, or on its own.
Hadlima can also be used to treat severe, active and progressive rheumatoid arthritis without previous methotrexate treatment.
Polyarticular Juvenile Idiopathic Arthritis (pJIA)
pJIA is an inflammatory disease of the joints. Hadlima is used to reduce the signs and symptoms of moderate to severely active pJIA, in patients 2 years of age and older, when other medicines are not appropriate. Your doctor will decide whether Hadlima should be used with another medicine called methotrexate or used alone.
Enthesitis-related arthritis (ERA)
ERA is an inflammatory disease of the joints and the places where tendons join the bone. Hadlima is used to treat ERA in children.
You may have already been given other medicines to treat your condition. Your doctor has prescribed Hadlima for you as you haven't responded well enough to these medicines.
Psoriatic arthritis (PsA)
PsA is an inflammatory disease of the joints that is usually associated with psoriasis. Signs and symptoms include joint pain, tenderness and swelling. Hadlima is used to reduce the signs and symptoms, of moderate to severely active PsA, as well as to slow down and protect the joints from further damage, to help them move more freely.
You may have already been given other medicines to treat your condition. Your doctor has prescribed Hadlima for you as you haven't responded well enough to these medicines.
Ankylosing spondylitis
Ankylosing spondylitis is an inflammatory disease of the spine. Signs and symptoms of ankylosing spondylitis include back pain and stiffness. Hadlima is used to reduce the signs and symptoms in patients with active disease.
Crohn's Disease
Crohn's disease is an inflammatory disease of the digestive tract. Hadlima is used to treat moderate to severe Crohn's disease in adults and children aged 6 years and over to reduce the signs and symptoms of the disease and to induce and maintain periods where the symptoms are no longer present (remission).
You may have already been given other medicines to treat your condition. Your doctor has prescribed Hadlima for you as you may either have not responded well enough, or you may have lost response or cannot tolerate these medicines.
Ulcerative Colitis
Ulcerative colitis is an inflammatory disease of the large intestine (bowel).
Hadlima is used to treat moderate to severe ulcerative colitis when other medicines are not appropriate.
Psoriasis
Psoriasis is an inflammatory disease of the skin. Plaque psoriasis, the most common form, is a skin condition that causes red, flaky, crusty patches of the skin covered with silvery scales. Plaque psoriasis can also affect nails, causing them to crumble, thicken and lift away from the nail bed which can be painful. Hadlima is used to treat moderate to severe forms of the disease in adults and severe forms in children and adolescents from 4 years of age for whom topical therapy (such as creams, lotions and ointments) and phototherapy (also known as light therapy) have either not worked very well, or are not suitable.
Hidradenitis suppurativa (HS)
HS (sometimes called acne inversa) is a chronic and often painful inflammatory skin disease.
Symptoms may include tender nodules (lumps) and abscesses (boils) that may leak pus, which can have an unpleasant odour. It most commonly affects specific areas of the skin, such as under the breasts, the armpits, inner thighs, groin and buttocks. Scarring may also occur in affected areas.
Hadlima is used for the treatment of adults and adolescents from 12 years of age with active moderate to severe HS, Hadlima can reduce the number of nodules and abscesses caused by the disease, and the pain that is often associated with it.
You may have already been given other medicines to treat your condition. Your doctor has prescribed Hadlima for you as you haven't responded well enough to these medicines.
Your doctor will schedule follow-up appointments to check on your progress to determine whether you should continue treatment.
Uveitis
Uveitis is an inflammatory disease affecting certain parts of the eye. This inflammation may lead to a decrease of vision and/or the presence of floaters in the eye (black dots or wispy lines that move across the field of vision). Hadlima is used to treat non-infectious intermediate, posterior and pan-uveitis.
You may have already been given other medicines to treat your condition. Your doctor has prescribed Hadlima for you as you may either have not responded well enough, or you have lost response or cannot tolerate these medicines.
The active ingredient in this medicine is adalimumab, which is a fully human monoclonal antibody. Monoclonal antibodies are proteins made by a type of blood cell to fight a foreign protein in the body.
Adalimumab recognises and binds to a specific protein (tumour necrosis factor or TNF-alpha), which is present at higher levels in some inflammatory diseases.
Ask your doctor if you have any questions about why this medicine has been prescribed for you.
This medicine is not addictive.
This medicine is only available with a doctor's prescription.
| 2. What should I know before I use Hadlima? |
Warnings
Do not use Hadlima if:
- you are allergic to adalimumab, or any of the ingredients listed at the end of this leaflet. Symptoms of an allergic reaction may include:
- chest tightness
- shortness of breath, wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body
- hives, itching or skin rash
- you have a severe infection such as sepsis (a serious infection of the blood) or tuberculosis (a serious infection of the lungs caused by bacteria), or other severe infection caused by a virus, fungus, parasite or bacteria.
- you have heart failure considered by your doctor to be moderate or severe.
Check with your doctor if you:
- have or have had an infection that does not go away or keeps coming back, this can include leg ulcers
- you have ever had tuberculosis, or you have been in close contact with someone who has tuberculosis. Tuberculosis can develop during therapy even if you have received treatment for the prevention of tuberculosis.
- you currently have active hepatitis B, have ever had hepatitis B, are a carrier of the hepatitis B virus or you think you may be at risk of contracting hepatitis B
- you have or have had an infection caused by a fungus, or you have lived or travelled in countries where fungal infections are common
- you have or have had uveitis, where the middle layer of the eyeball is inflamed
- you have or have had allergic reactions such as chest tightness, wheezing, dizziness, swelling or rash
- you have a disease that affects the insulating layer of the nerves, e.g. multiple sclerosis (MS)
- you have or have had a blood disorder
- you have or have had low resistance to disease
- you have or have had a heart condition
- you have or have had cancer or autoimmune disease
- you have a lung disease called chronic obstructive pulmonary disease (COPD)
- you have or have had kidney or liver problems
- you have any vaccinations scheduled
- you have or have had psoriasis (a skin disease that produces patches of thickened, scaly skin that is not contagious)
- you have had phototherapy, also known as light therapy, for psoriasis
- you have any surgery planned
- you take any medicines for any other condition.
During treatment, you may be at risk of developing certain side effects. It is important you understand these risks and how to monitor for them. See additional information under Section 6. Are there any side effects?
Pregnancy and breastfeeding
Make sure your doctor is aware that you are pregnant or plan to become pregnant. Hadlima should only be used in pregnancy if clearly needed.
If you use Hadlima during pregnancy your baby may have a higher risk of getting an infection.
You should consider the use of effective contraception to prevent pregnancy and continue its use for at least 5 months after the last Hadlima injection.
Tell your baby's doctors if you have taken Hadlima while you are pregnant, especially before your baby receives any vaccinations.
Make sure your doctor is aware that you are breastfeeding, or you plan to do so.
Use in children
- Wherever possible, it is recommended that children are up to date with all vaccinations, according to current immunisation guidelines, before they are started on Hadlima treatment.
- Treatment of Crohn's disease in children should be supported by good nutrition to allow appropriate growth.
- The long-term effects of Hadlima on the growth and development of children is not known.
Use in the elderly
- If you are over 65, you may be more likely to get an infection while taking Hadlima.
| 3. What if I am taking other medicines? |
Tell your doctor or pharmacist if you are taking any other medicines, including any medicines, vitamins or supplements that you buy without a prescription from your pharmacy, supermarket or health food shop.
Some medicines may interfere with Hadlima and affect how it works, while Hadlima may affect how other medicines work.
Do not take Hadlima if you are taking the following medicine:
- anakinra, a medicine used to treat rheumatoid arthritis, juvenile idiopathic arthritis and conditions associated with a defect in a protein called cryoprin.
Medicines that may increase the risk of infection when taken with Hadlima include:
- anakinra, a medicine used to treat rheumatoid arthritis,
- juvenile idiopathic arthritis and conditions associated with a defect in a protein called cryoprin
- abatacept, a medicine used to treat rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, psoriatic arthritis
- azathioprine, a medicine used for suppressing the immune system to treat various conditions
- 6-mercaptopurine, a medicine used to treat certain types of leukaemia, a blood disorder.
Check with your doctor or pharmacist if you are not sure about what medicines, vitamins or supplements you are taking and if these affect Hadlima.
| 4. How do I use Hadlima? |
How much to use
- Rheumatoid arthritis in adults
Inject one 40 mg dose every fortnight.
If you are not taking methotrexate, your doctor may change this dose to 40 mg every week, or 80 mg every fortnight, depending on your response. - Psoriatic arthritis and ankylosing spondylitis in adults
Inject one 40 mg dose every fortnight. - Crohn's disease and Ulcerative Colitis in adults
Inject 160 mg on day 1, followed by 80 mg on day 15 and 40 mg on day 29. Then, continue to inject 40 mg every fortnight (maintenance dose).
Your doctor may change this maintenance dose to 40 mg every week, or 80 mg every fortnight, depending on your response.
(See Special dosing instructions at the end of this section.) - Psoriasis in adults
Inject 80 mg on day 1, followed by 40 mg on day 8 and 40 mg on day 22. Then, continue to inject 40 mg every fortnight (maintenance dose).
Your doctor may change this maintenance dose to 40 mg every week, or 80 mg every fortnight, depending on your response.
(See Special dosing instructions at the end of this section.) - Uveitis in adults
Inject 80 mg on day 1, followed by 40 mg on day 8 and 40 mg on day 22. Then continue to inject 40 mg every fortnight (maintenance dose).
(See Special dosing instructions at the end of this section.) - Hidradenitis suppurativa in adults
Inject 160 mg on day 1, followed by 80 mg on day 15. Then continue to inject 40 mg every week or 80mg every fortnight from day 29 (maintenance dose).
(See Special dosing instructions at the end of this section.) - Polyarticular juvenile idiopathic arthritis and enthesitis-related arthritis
If the patient's body weight is at least 30 kg, inject one 40 mg dose every fortnight.
If the patient's body weight is between 10 and 30 kg, inject one 20 mg dose every fortnight. - Crohn's disease in children
If the patient's body weight is at least 40 kg, inject 160 mg on day 1, followed by 80 mg on day 15 and 40 mg on day 29. Then, continue to inject 40 mg every fortnight (maintenance dose).
Your doctor may change this maintenance dose to 40 mg every week, or 80 mg every fortnight, depending on your response.
(See Special dosing instructions at the end of this section.) - Psoriasis in children
If the patient's body weight is at least 40 kg, inject 40 mg on day 1, followed by 40 mg on day 8 and 40 mg on day 22. Then continue to inject 40 mg every fortnight (maintenance dose). - Hidradenitis suppurativa (HS) in adolescents
Inject 80 mg on day 1, followed by 40 mg on day 8, and 40 mg on day 22. Then continue to inject 40 mg every fortnight (maintenance dose)
Your doctor may change this maintenance dose to 40 mg every week, or 80 mg every fortnight depending on your response
(See Special dosing instructions at the end of this section.)
Use an antiseptic face wash on the affected areas.
Special dosing instructions
- When a dose of 160 mg is required, this can be given as four 40 mg injections in one day, or two 40 mg injections per day over two consecutive days.
- When a dose of 80 mg is required, this can be given as two 40 mg injections in one day.
In some instances, Hadlima needs to be taken with other medicines. Your doctor will let you know which medicines, how to take them and how long to take them.
Follow all instructions given to you and use Hadlima until your doctor tells you to stop.
How to use Hadlima
- Hadlima is injected under the skin (sub-cutaneous injection).
- It can be injected by the patient, or by someone else, such as a family member, friend or carer.
- An injection should not be attempted until proper training has been received on the correct injection technique.
- Do not mix the injection with any other medicine.
- If you are using the Hadlima PushTouch auto-injector, instructions for preparing and giving an injection are provided on the Product Information/Instruction for use leaflet with the product.
If you forget to use Hadlima
It is important that you use your medicine as prescribed by your doctor.
If you miss your dose at the usual time, inject Hadlima as soon as you remember, and continue injecting the next dose at the usual time on your scheduled day.
Do not take a double dose to make up for the dose you missed.
If you use too much Hadlima
You should immediately:
- phone the Poisons Information Centre
(by calling 13 11 26), or - contact your doctor, or
- go to the Emergency Department at your nearest hospital.
You should do this even if there are no signs of discomfort or poisoning.
| 5. What should I know while using Hadlima? |
Things you should do
- Keep all your doctor's appointments so your progress can be tracked.
- Keep your appointments for blood tests. Some side effects are seen in blood results before you have any symptoms.
- Remind any doctor, dentist or pharmacist you visit that you are using Hadlima, especially if you are scheduled for surgery or to receive any live vaccines (e.g. Bacille Calmette-Guerin or oral polio vaccine).
Call your doctor straight away if you:
- get symptoms of an infection, such as a fever, skin sores, feeling tired, any problems with your teeth or gums or pain when passing urine or blood in your urine.
- become pregnant while using Hadlima
- notice new skin lesions (skin spots or sores), or if existing lesions change appearance.
Things you should not do
Do not stop taking Hadlima, without checking with your doctor.
Driving or using machines
Be careful driving or operating machinery until you know how Hadlima affects you.
Drinking alcohol
There is no information on the use of alcohol with Hadlima.
Looking after your medicine
Follow the instructions in the carton on how to take care of your medicine properly.
- Keep Hadlima in the carton protected from light.
- Keep Hadlima in a refrigerator (at 2°C to 8°C). Do not freeze.
Keep it where young children cannot reach it.
When to discard your medicine
- When necessary, a single Hadlima PushTouch auto-injector 40 mg in 0.8 mL may be stored at room temperature (25°C) for a maximum of 28 days, protected from light. Once removed from the refrigerator, the PushTouch auto-injector 40 mg in 0.8 mL must be used within 28 days or discarded, even if it has been returned to the refrigerator.
- When necessary, a single Hadlima PushTouch auto-injector 40 mg in 0.4 mL may be stored at room temperature (25°C) for a maximum of 31 days, protected from light. Once removed from the refrigerator, the PushTouch auto-injector 40 mg in 0.4 mL must be used within 31 days or discarded, even if it has been returned to the refrigerator.
- After injecting Hadlima, immediately throw away the used PushTouch auto-injector in a special sharps container as instructed by your doctor, nurse or pharmacist.
Getting rid of any unwanted medicine
If your doctor advises that you no longer need to use this medicine or it is out of date, follow local guidelines for safe disposal.
| 6. Are there any side effects? |
All medicines can have side effects. If you do experience any side effects, most of them are minor and temporary. However, some side effects may need medical attention.
See the information below and, if you need to, ask your doctor or pharmacist if you have any further questions about side effects.
Less serious side effects
| Less serious side effects | What to do |
Injection site:
| Speak to your doctor if you have any of these less serious side effects and they worry you. |
Serious side effects
| Serious side effects | What to do |
Signs of tuberculosis, such as:
| Speak to your doctor as soon as possible if you have any of these side effects. |
Very serious side effects
| Very serious side effects | What to do |
Signs of an allergic reaction, such as:
| Call your doctor straight away or go straight to the Emergency Department at your nearest hospital if you notice any of these serious side effects. |
Tell your doctor or pharmacist if you notice anything else that may be making you feel unwell.
Other side effects not listed here may occur in some people.
Reporting side effects
After you have received medical advice for any side effects you experience, you can report side effects to the Therapeutic Goods Administration online at www.tga.gov.au/reporting-problems. By reporting side effects, you can help provide more information on the safety of this medicine.
Always make sure you speak to your doctor or pharmacist before you decide to stop taking any of your medicines.
| 7. Product details |
This medicine is only available with a doctor's prescription.
What Hadlima contains
Hadlima 40 mg in 0.8 mL (50 mg/mL):
40 mg adalimumab in 0.8 mL solution in a syringe available in a PushTouch auto-injector (AUST R 284248)
| Active ingredient (main ingredient) | adalimumab |
| Other ingredients (inactive ingredients) | Sodium citrate dihydrate Citric acid monohydrate Histidine Histidine hydrochloride monohydrate Sorbitol Polysorbate 20 Water for injections |
Hadlima 40 mg in 0.4 mL (100 mg/mL):
40 mg adalimumab in 0.4 mL solution in a PushTouch auto-injector (AUST R 384975)
| Active ingredient (main ingredient) | adalimumab |
| Other ingredients (inactive ingredients) | Histidine Histidine hydrochloride monohydrate Mannitol Polysorbate 20 Dibasic sodium phosphate heptahydrate Monobasic sodium phosphate monohydrate Sodium succinate Succinic acid Water for injections |
What Hadlima looks like
Hadlima is a clear to opalescent, colourless to pale brown, sterile solution of 40 mg adalimumab in 0.8 mL or 0.4 mL solution in a syringe available in a PushTouch auto-injector for patient use in packs containing 2 PushTouch auto-injectors.
Who distributes Hadlima
Hadlima is distributed in Australia by:
Sponsor:
Samsung Bioepis AU Pty Ltd
Suite 1, Level 11, 66 Goulburn Street,
Sydney NSW 2000, Australia
Distributor:
Organon Pharma Pty Limited
Building A, 26 Talavera Road,
Macquarie Park, NSW 2113, Australia
This leaflet was prepared in:
December 2023
Version 9.0
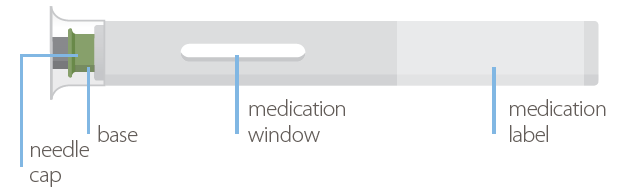
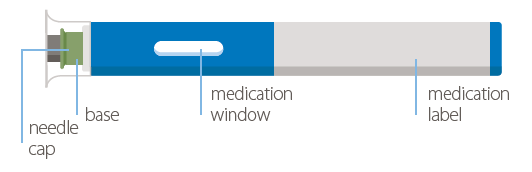
| Instructions for Use: |
Your single-dose auto-injector (40 mg/0.8 mL):
- There is no button on your auto-injector!
- The needle is hidden below the green base. When you push the device firmly onto your skin, the injection will start automatically.
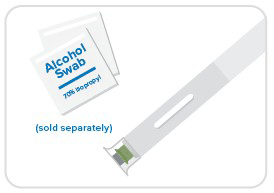
Step 1: Gather supplies
- Place your auto-injector and unopened alcohol swabs on a clean, dry surface.
- Remember to wash your hands!
- Don't remove the cap just yet!
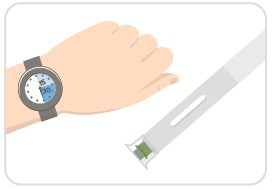
Step 2: Wait 15-30 minutes
- Wait 15-30 minutes for your auto-injector to warm-up to room temperature, which helps to reduce pain during injection.
- Don't remove the cap just yet!
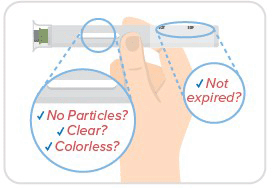
Step 3: Inspect medicine & date
- Always make sure your medicine is clear to opalescent, free of particles, and colorless to pale brown and hasn't expired.
- You may see 1 or more air bubbles, and that's okay.
- Don't remove the cap just yet!
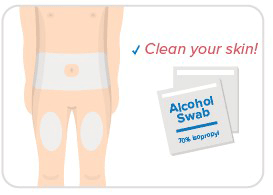
Step 4: Choose site & clean skin
- Choose an injection site on your body.
- Your abdomen or thighs are best.
- Wipe the injection site with an alcohol swab.
- Avoid skin that's sore, bruised, scarred, scaly, or has red patches.
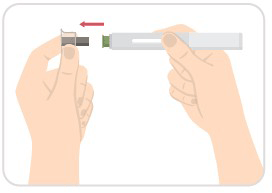
Step 5: Pull off the clear needle cap
- Carefully pull off the clear needle cap with a metal center from the auto-injector.
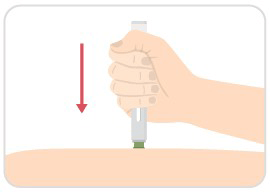
Step 6: Place green base, press down, and hold
- Place the green base straight on your skin, and push the entire device down firmly to start the injection.
- When you push down, the injection starts.
- You may hear a first click.
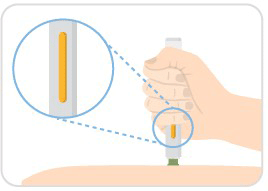
Step 7: Continue to hold
- Hold the auto-injector against your skin until the yellow indicator fills the medication window and stops moving.
- Several seconds later you may hear a second click.
Step 8: Confirm completion & dispose auto-injector
- Confirm that the entire medication window is yellow.
- Discard your auto-injector in a sharps container.

Your single-dose auto-injector (40 mg/0.4 mL):
- There is no button on your auto-injector!
- The needle is hidden below the green base. When you push the device firmly onto your skin, the injection will start automatically.
Step 1: Gather supplies
- Place your auto-injector and unopened alcohol swabs on a clean, dry surface.
- Remember to wash your hands!
- Don't remove the cap just yet!

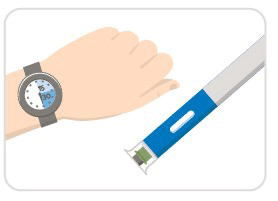
Step 2: Wait 15-30 minutes
- Wait 15-30 minutes for your auto-injector to warm-up to room temperature, which helps to reduce pain during injection.
- Don't remove the cap just yet!
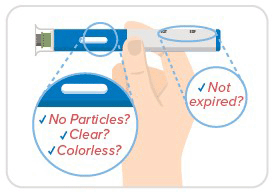
Step 3: Inspect medicine & date
- Always make sure your medicine is clear to opalescent, free of particles, and colorless to pale brown and hasn't expired.
- You may see 1 or more bubbles, and that's okay.
- Don't remove the cap just yet!
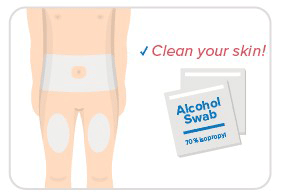
Step 4: Choose site & clean skin
- Choose an injection site on your body.
- Your abdomen or thighs are best.
- Wipe the injection site with an alcohol swab.
- Avoid skin that's sore, bruised, scarred, scaly, or has red patches.
Step 5: Pull off the clear needle cap
- Carefully pull off the clear needle cap with a metal center from the auto-injector.
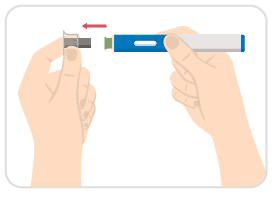
Step 6: Place green base, press down, and hold
- Place the green base straight on your skin, and push the entire device down firmly to start the injection.
- When you push down, the injection starts.
- You may hear a first click.
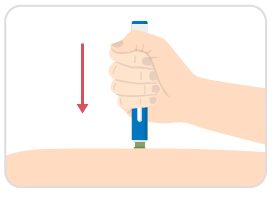
Step 7: Continue to hold
- Hold the auto-injector against your skin until the yellow indicator fills the medication window and stops moving.
- Several seconds later you may hear a second click.
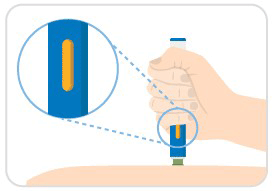
Step 8: Confirm completion & dispose auto-injector
- Confirm that the entire medication window is yellow.
- Discard your auto-injector in a sharps container.

Published by MIMS February 2024
