IKERVIS®
| Consumer Medicine Information (CMI) summary |
The full CMI on the next page has more details. If you are worried about using this medicine, speak to your doctor or pharmacist.
| ▼ This medicine is new or being used differently. Please report side effects. See the full CMI for further details. |
| 1. Why am I using IKERVIS®? |
IKERVIS® contains the active ingredient ciclosporin. IKERVIS® is used to treat severe keratitis in adult patients with dry eye disease which has not improved despite treatment with artificial tears.
For more information, see Section 1. Why am I using IKERVIS®? in the full CMI.
| 2. What should I know before I use IKERVIS®? |
Do not use if you have ever had an allergic reaction to IKERVIS® or any of the ingredients listed at the end of the CMI.
Talk to your doctor if you have any other medical conditions, take any other medicines, or are pregnant or plan to become pregnant or are breastfeeding.
For more information, see Section 2. What should I know before I use IKERVIS®? in the full CMI.
| 3. What if I am taking other medicines? |
Some medicines may interfere with IKERVIS® and affect how it works.
A list of these medicines is in Section 3. What if I am taking other medicines? in the full CMI.
| 4. How do I use IKERVIS®? |
- The usual dose of IKERVIS® is one drop, once daily, to be applied to the affected eye(s) at bedtime.
- Follow all directions given to you by your doctor, optometrist or pharmacist carefully. They may differ from the information contained in this leaflet.
More instructions can be found in Section 4. How do I use IKERVIS®? in the full CMI.
| 5. What should I know while using IKERVIS®? |
| Things you should do |
|
| Things you should not do |
|
| Driving or using machines |
|
| Looking after your medicine |
|
For more information, see Section 5. What should I know while using IKERVIS®? in the full CMI.
| 6. Are there any side effects? |
The most common side effects are eye pain and eye irritation. These side effects are usually temporary and associated with administering the drops to the eye.
For more information, including what to do if you have any side effects, see Section 6. Are there any side effects? in the full CMI.
| ▼ This medicine is subject to additional monitoring. This will allow quick identification of new safety information. You can help by reporting any side effects you may get. You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems. |
IKERVIS®
Active ingredient(s): Ciclosporin
| Consumer Medicine Information (CMI) |
This leaflet provides important information about using IKERVIS®. You should also speak to your doctor, optometrist or pharmacist if you would like further information or if you have any concerns or questions about using IKERVIS®.
Where to find information in this leaflet:
1. Why am I using IKERVIS®?
2. What should I know before I use IKERVIS®?
3. What if I am taking other medicines?
4. How do I use IKERVIS®?
5. What should I know while using IKERVIS®?
6. Are there any side effects?
7. Product details
| 1. Why am I using IKERVIS®? |
IKERVIS® contains the active ingredient ciclosporin. Ciclosporin belongs to a group of medicines known as immunosuppressive agents that are used to reduce inflammation.
IKERVIS is used to treat adults with severe keratitis (inflammation of the cornea, the transparent layer in the front part of the eye) as a result of dry eye disease, which has not improved despite treatment with tear substitutes (artificial tears).
| 2. What should I know before I use IKERVIS®? |
Warnings
Do not use IKERVIS® if:
- you are allergic to ciclosporin, or any of the ingredients listed at the end of this leaflet.
Always check the ingredients to make sure you can use this medicine. - you have or have had a cancer in or around your eye
- you have an eye infection.
Check with your doctor if you:
- have any other medical conditions, in particular, any conditions which affect your eyes
- take medicines for any other condition, particularly medicines that belong to a group called steroids or medicines used to treat glaucoma
- if you have previously had an eye infection caused by the herpes virus that might have damaged the transparent front part of the eye (cornea).
During treatment, you may be at risk of developing certain side effects. It is important you understand these risks and how to monitor for them. See additional information under Section 6. Are there any side effects?
Contact Lenses
Contact lenses can further damage the transparent front part of the eye (cornea). Therefore, you should remove your contact lenses at bedtime before using IKERVIS; you can reinsert them when you wake up.
Pregnancy and breastfeeding
Check with your doctor if you are pregnant or intend to become pregnant.
IKERVIS® should not be used if you are pregnant or planning to become pregnant.
Talk to your doctor if you are breastfeeding or intend to breastfeed.
It is not known if IKERVIS® passes into breast milk.
Adolescent and children under 18 years.
IKERVIS® should not be used in children and adolescents under the age of 18 years.
| 3. What if I am taking other medicines? |
Tell your doctor, optometrist or pharmacist if you are taking any other medicines, including any medicines, vitamins or supplements that you buy without a prescription from your pharmacy, supermarket or health food shop.
Co-administration of IKERVIS® with eye drops containing corticosteroids could increase the effects of ciclosporin on the immune system. This could increase the risk of side effects.
IKERVIS® eye drops should be used at least 5 minutes after any other eye drops are used.
Check with your doctor, optometrist or pharmacist if you are not sure about what medicines, vitamins or supplements you are taking and if these affect IKERVIS®.
| 4. How do I use IKERVIS®? |
How much to use
- The usual dose of IKERVIS® is one drop in each affected eye, once daily at bedtime.
- Follow the instructions provided and use IKERVIS® until your doctor or optometrist tells you to stop.
When to use IKERVIS®
- IKERVIS® should be used once daily at bedtime.
How to use IKERVIS®
Follow these instructions carefully and ask your doctor or pharmacist if there is anything you do not understand.
- Wash your hands.
- If you wear contact lenses, take them out before using the drops; you can reinsert them when you wake up.
- Open one of the foil pouches. Each pouch contains 5 single-use containers (ampoules).
- Take one single-use container from the foil pouch.
- Gently shake the container prior to use.
- Twist off the cap.
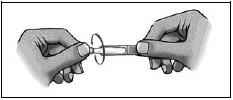
- Pull down your lower eyelid.
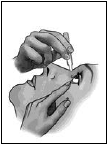
- Tilt your head back and look up at the ceiling.
- Gently squeeze one drop of the medicine onto your eye. Make sure you do not touch your eye with the tip of the single-use container.
- Blink a few times so that the medicine covers your eye.
- After using IKERVIS®, press a finger into the corner of your eye by the nose and gently close your eyelids for 2 minutes. This helps to stop IKERVIS® getting into the rest of the body.
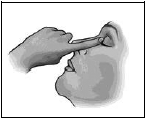
- If you use drops in both eyes, repeat the steps for your other eye.
- Discard the single-use container as soon as you have used it, even if there is still some liquid left in it.
- The remaining single-use containers should be kept in the foil pouch.
If a drop misses your eye, try again.
If you use more IKERVIS® than you should, rinse your eye with water. Do not put in any more drops until it is time for your next regular dose.
If you forget to use IKERVIS®
IKERVIS® should be used regularly at approximately the same time each day. If you forget to use IKERVIS at bedtime, wait until the next dose as planned. Do not use a double dose to make up for the forgotten dose. Do not use more than one drop each day in the affected eye(s).
If you are not sure what to do, ask your doctor, optometrist or pharmacist.
If you stop using IKERVIS® without speaking to your doctor or optometrist, the inflammation of the transparent front part of your eye (known as keratitis) may not be controlled and could lead to impaired vision.
If you use too much IKERVIS®
If you think that you have used too much IKERVIS®, immediately rinse your eye(s) with water.
If you think that you or anyone else may have swallowed any or all of the IKERVIS® eye drops, immediately:
- phone the Poisons Information Centre
(by calling 13 11 26), or - contact your doctor, or
- go to the Emergency Department at your nearest hospital.
You should do this even if there are no signs of discomfort or poisoning.
| 5. What should I know while using IKERVIS®? |
Things you should do
- Tell your doctor immediately if you become pregnant.
Remind any doctor, dentist, pharmacist or optometrist you visit that you are using IKERVIS®.
Things you should not do
- Do not stop using this medicine without checking with your doctor or optometrist.
- Do not give IKERVIS® to anyone else, even if they have the same condition as you.
Driving or using machines
Be careful before you drive or use any machines or tools until you know how IKERVIS® affects you.
Your vision may be blurred immediately after using IKERVIS® eye drops. If this happens, wait until your vision clears before you drive or use machines.
Looking after your medicine
Follow the instructions in the carton on how to take care of your medicine properly.
- Keep the IKERVIS® single-dose containers in the foil pouch until it is time to use them. This is important to protect the product from light and to avoid evaporation.
- Keep the pack in a cool dry place where the temperature stays below 30°C.
- Do not freeze.
Store the pack in a cool dry place away from moisture, heat or sunlight; for example, do not store it:
- in the bathroom or near a sink, or
- in the car or on windowsills.
Keep the pack where young children cannot reach it.
When to discard your medicine
Discard any opened individual single-dose container with any remaining emulsion immediately after use.
Getting rid of any unwanted medicine
If you no longer need to use this medicine or it is out of date, take it to any pharmacy for safe disposal.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
Do not use this medicine after the expiry date.
| 6. Are there any side effects? |
All medicines can have side effects. If you do experience any side effects, most of them are mild to moderate and temporary. However, some side effects may need medical attention.
See the information below and, if you need to, ask your doctor, optometrist or pharmacist if you have any further questions about side effects.
Less serious side effects
| Less serious side effects | What to do |
Eyes - related:
| Speak to your doctor or optometrist if you have any of these less serious side effects and they worry you. |
Serious side effects
| Serious side effects | What to do |
Eyes - related:
These side effects may be signs of an allergic reaction. | Call your doctor or optometrist straight away, or go straight to the Emergency Department at your nearest hospital if you notice any of these serious side effects. |
Tell your doctor, optometrist or pharmacist if you notice anything else that may be making you feel unwell.
Other side effects not listed here may occur in some people.
Reporting side effects
After you have received medical advice for any side effects you experience, you can report side effects to the Therapeutic Goods Administration online at www.tga.gov.au/reporting-problems. By reporting side effects, you can help provide more information on the safety of this medicine.
Always make sure you speak to your doctor or pharmacist before you decide to stop taking any of your medicines.
| 7. Product details |
This medicine is only available with a doctor's prescription.
What IKERVIS® contains
| Active ingredient (main ingredient) | Ciclosporin. |
| Other ingredients (inactive ingredients) | Medium chain triglycerides Cetalkonium chloride Glycerol Poloxamer Sodium hydroxide Water for injections |
Do not take this medicine if you are allergic to any of these ingredients.
IKERVIS® does not contain lactose, sucrose, gluten, tartrazine or any other azo dyes.
What IKERVIS® looks like
IKERVIS® is a milky white eye drop emulsion.
It is supplied in single-dose containers made of a low-density polyethylene (LDPE).
Each single-dose container contains 0.3 mL of the eye drop emulsion.
A strip of 5 single-dose containers is wrapped in a sealed aluminium foil pouch.
Each box of IKERVIS® contains 30 single-dose containers (6 foil pouches each containing a strip of 5 single-dose containers).
Aust R 319502.
Who distributes IKERVIS®
Seqirus Pty Ltd
ABN 26 160 735 035
63 Poplar Road,
Parkville, VIC, 3052
Australia
Telephone: 1800 642 865
www.seqirus.com.au
IKERVIS is a registered trademark of Santen SAS.
This leaflet was prepared in August 2021.
Published by MIMS September 2021
