What is in this leaflet
This leaflet answers some common questions about Jardiamet.
It does not contain all the available information.
It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking Jardiamet against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
This leaflet was last updated on the date at the end of this leaflet. More recent information may be available. The latest Consumer Medicine Information is available from your pharmacist, doctor, or from www.medicines.org.au and may contain important information about the medicine and its use of which you should be aware.
Keep this leaflet with the medicine. You may need to read it again.
What Jardiamet is used for
Jardiamet is used to lower blood sugar levels in patients with type 2 diabetes mellitus.
It may be used when diet plus exercise do not provide adequate blood sugar level control either:
- alone as a single medicine, or
- in combination with certain other anti-diabetic medicines such as:
- sulfonylurea
- pioglitazone
- insulin
- linagliptin.
If you have type 2 diabetes mellitus and cardiovascular disease, empagliflozin (one of the active ingredients in Jardiamet) can be used to reduce your risk of dying from your cardiovascular disease.
Type 2 diabetes mellitus is also called non-insulin-dependent diabetes mellitus or NIDDM. Type 2 diabetes develops if the body does not make enough insulin, or if the insulin that your body makes does not work as well as it should.
Insulin is a substance which helps to lower the level of sugar in your blood, especially after meals.
When the level of sugar builds up in your blood, this can cause damage to the body's cells and lead to serious problems with your heart, eyes, circulation or kidneys.
How Jardiamet works
Jardiamet contains two different active ingredients:
- empagliflozin, which belongs to a group of medicines called SGLT2 (sodium-glucose co-transporter 2) inhibitors, and
- metformin, which belongs to a class of medicines called biguanides.
Both medicines work together to control blood sugar levels in patients with type 2 diabetes mellitus by increasing the amount of glucose expelled in urine, and lowering the amount of sugar made by your body.
Lowering and controlling blood sugar may help prevent or delay complications of diabetes, such as heart disease, kidney disease, blindness and foot amputation.
Along with diet and exercise, this medicine helps lower your blood sugar.
Your doctor may have prescribed Jardiamet to replace the anti-diabetic medicine(s) you are currently taking. It is important that you continue to follow the diet and/or exercises recommended for you while you are on treatment with Jardiamet.
Ask your doctor if you have any questions about why this medicine has been prescribed for you. Your doctor may have prescribed it for another reason.
This medicine is available only with a doctor's prescription. It is not addictive.
Before you take Jardiamet
When you must not take it
Do not take Jardiamet if you have an allergy to:
- any medicine containing empagliflozin or metformin (the active ingredients in Jardiamet)
- any of the other ingredients listed at the end of this leaflet.
Some of the symptoms of an allergic reaction may include:
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body
- rash, itching or hives on the skin.
Do not take Jardiamet if you:
- have any type of metabolic acidosis such as lactic acidosis, diabetic ketoacidosis (a symptom of uncontrolled diabetes, in which substances called ketone bodies build up in the blood - you may notice this as an unusual fruity odour on your breath, difficulty breathing, confusion and frequent urination)
- have had a diabetic pre-coma
- have problems with your kidneys
- have problems with your liver
- have a severe infection
- are dehydrated
- are treated for acute heart failure or have recently had a heart attack
- have severe problems with your circulation (such as shock)
- have severe breathing difficulties
- have blood clots in the lungs (symptoms include coughing, shortness of breath, chest pain and a fast heart rate)
- have significant blood loss
- have gangrene
- have inflammation of the pancreas (pancreatitis), symptoms include severe upper stomach pain, often with nausea and vomiting
- drink excessive alcohol (all the time or “binge” drinking).
Talk to your doctor about when to stop taking Jardiamet and when to start taking it again if you:
- are going to have an X-ray where you will be injected with an iodinated contrast (dye)
- are planning to have surgery (including where the use of insulin is essential).
Do not take this medicine if you are pregnant or intending to become pregnant. It may affect your developing baby if you take it during pregnancy.
Do not breastfeed if you are taking this medicine. Metformin, one of the active ingredients in Jardiamet passes into human breast milk and could affect your baby. It is not known whether the active ingredient, empagliflozin passes into human breast milk.
Do not give this medicine to a child under the age of 18 years. Safety and effectiveness in children younger than 18 years has not been established.
Do not take this medicine after the expiry date printed on the pack or if the packaging is torn or shows signs of tampering. If it has expired or is damaged, return it to your pharmacist for disposal.
If you are not sure whether you should start taking this medicine, talk to your doctor.
Before you start to take it
Discard any other medicines containing metformin or empagliflozin that your doctor might have prescribed to you in the past and that you may still have in your possession. Jardiamet contains empagliflozin and metformin. If you have more than one metformin-containing medicine in your possession you may accidentally take too much (overdose). Accidentally taking too much metformin can cause a very serious side effect called lactic acidosis.
ACCIDENTAL METFORMIN OVERDOSING IS A SIGNIFICANT SAFETY RISK.
Ask your doctor or pharmacist if you are unsure if you have any other medicines containing metformin.
Metformin is sold under many different brand names in Australia.
Your doctor or pharmacist will know which other medicines also contain metformin.
Tell your doctor if you have allergies to any other medicines, foods, preservatives or dyes.
Tell your doctor if you have type 1 diabetes, a condition where your body does not produce insulin. Jardiamet should not be used to treat type 1 diabetes.
Tell your doctor if you are sick, have diarrhoea or fever, or if you are not able to eat or drink. These conditions can cause dehydration. Your doctor may ask you to stop taking Jardiamet until you recover to prevent loss of too much body fluid.
Tell your doctor if you have a serious infection of the kidney or the urinary tract with fever. Your doctor may ask you to stop taking Jardiamet until you have recovered.
Tell your doctor if you have heart problems, history of low blood pressure, or are 75 years of age or older. Increased passing of urine due to the medicine may affect fluid balance in your body and increase your risk of dehydration.
Tell your doctor if you are 85 years of age or older. You should not start taking Jardiamet if you are over 85 years of age.
If you have not told your doctor about any of the above, tell him/her before you start taking Jardiamet.
Taking other medicines
Tell your doctor or pharmacist if you are taking any other medicines, including any that you get without a prescription from your pharmacy, supermarket or health food shop.
Some medicines and Jardiamet may interfere with each other. These include:
- an antibiotic medicine used to treat certain infections such as tuberculosis (rifampicin)
- a medicine used to treat reflux and ulcers (cimetidine)
- medicines used to treat diseases that involve inflammation, like asthma and arthritis (corticosteroids)
- specific medicines for the treatment of high blood pressure (ACE inhibitors, angiotensin II receptor antagonists, calcium channel blockers, beta blockers)
- medicines used to prevent blood clots, such as warfarin
- medicines which increase urine production (diuretics)
- some medicines used to treat asthma (salbutamol or terbutaline)
- medicines used to relieve pain, swelling and other symptoms of inflammation (NSAIDs (non-steroidal anti-inflammatory drugs) such as aspirin, diclofenac, ibuprofen, meloxicam, naproxen or piroxicam, and selective COX II inhibitors such as celecoxib, parecoxib, etoricoxib)
- iodinated contrast agents (which you may receive while having an X-ray)
- alcohol-containing medicines
- a medicine used in people with multiple sclerosis, and in young children to treat some types of seizures (fits) (tetracosactrin)
- a medicine used to treat endometriosis (danazol)
- a medicine used to treat schizophrenia and other mental illnesses (chlorpromazine)
- a medicine used to treat and prevent mood disorders (lithium)
- medicines used in the treatment of HIV and chronic hepatitis C infections (dolutegravir, daclatasvir)
- medicines used in the treatment of certain cancers (crizotinib, olaparib, vandetanib)
- medicines used to control fits (seizures), chronic pain or glaucoma (topiramate, zonisamide, acetazolamide, dichlorphenamide).
These medicines may be affected by Jardiamet or may affect how well it works. You may need different amounts of your medicines, or you may need to take different medicines.
Your doctor, pharmacist or diabetes educator can tell you what to do if you are taking any of these medicines. They also have more information on medicines to be careful with or avoid while taking this medicine.
How to take Jardiamet
Follow all directions given to you by your doctor or pharmacist carefully. They may differ from the information contained in this leaflet.
If you do not understand the instructions on the box, ask your doctor or pharmacist for help.
How much to take
Your doctor will tell you how many Jardiamet tablets to take and how often you should take them.
The usual dose is one Jardiamet tablet twice daily.
Take Jardiamet exactly as your doctor or pharmacist has told you.
How to take it
Swallow the tablets whole with a full glass of water during or after meals. This will lessen the chance of a stomach upset.
When to take it
Take your medicine at about the same time each day. Taking it at the same time each day will have the best effect. It will also help you remember when to take it.
How long to take it
It is important that you take Jardiamet every day.
Continue taking your medicine for as long as your doctor tells you.
This medicine helps to control your condition, but does not cure it. It is important to keep taking your medicine even if you feel well.
If you forget to take it
If it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to.
Otherwise, take it as soon as you remember, and then go back to taking your medicine as you would normally.
Do not take a double dose to make up for the dose that you missed. This may increase the chance of you getting an unwanted side effect.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering to take your medicine, ask your pharmacist for some hints.
If you take too much (overdose)
Immediately telephone your doctor or the Poisons Information Centre (telephone 13 11 26) for advice, or go to Emergency at the nearest hospital, if you think that you or anyone else may have taken too much Jardiamet. Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
While you are taking Jardiamet
Things you must do
If you are about to be started on any new medicine, remind your doctor and pharmacist that you are taking Jardiamet.
Tell any other doctors, dentists, and pharmacists who treat you that you are taking this medicine.
Talk to your doctor about when to stop taking Jardiamet and when to start taking it again if you are about to have surgery or an examination such as an X-ray or scan requiring an injection of iodinated contrast (dye).
If you are intending to become pregnant or are pregnant talk to your doctor about alternative medications to control your blood glucose level. It is important your blood glucose levels are as close to normal as possible at this time.
If you are about to have any blood tests, tell your doctor that you are taking this medicine. It may interfere with the results of some tests.
Keep all of your doctor's appointments so that your progress can be checked. Your doctor may want to perform blood tests to check your kidneys and vitamin B12 levels while you are taking Jardiamet.
Follow your doctor's and/or dietician's advice on diet, drinking alcohol and exercise. Diet and exercise can help your body use its blood sugar better. It is important to stay on the diet and exercise program recommended by your doctor while taking Jardiamet.
Make sure you check your blood glucose regularly. This is the best way to tell if your diabetes is being controlled properly. Your doctor or diabetes educator will show you how and when to do this.
Check your feet regularly and see your doctor if you notice any problems. Follow any other advice regarding foot care given by your doctor.
Tell your doctor if you become ill or dehydrated, or experience stress, injury, fever, infection, or need surgery. Your blood glucose may become difficult to control at these times. You may also be at greater risk of developing a serious condition called lactic acidosis or diabetic ketoacidosis. During these times, your doctor may temporarily replace Jardiamet with insulin.
Make sure that you, your friends, family and work colleagues can recognise the symptoms of hypoglycaemia and hyperglycaemia and know how to treat them.
HYPOGLYCAEMIA
Jardiamet does not normally cause hypoglycaemia, although you may experience it if you take certain other medicines.
Signs of hypoglycaemia may include:
- weakness, trembling or shaking
- sweating
- light-headedness, dizziness, headache or lack of concentration
- irritability, tearfulness or crying
- hunger
- numbness around the lips and tongue.
If not treated quickly, these symptoms may progress to:
- loss of co-ordination
- slurred speech
- confusion
- fits or loss of consciousness.
At the first signs of hypoglycaemia, you need to raise your blood glucose quickly.
You can do this by taking one of the following:
- 5-7 jelly beans
- 3 teaspoons of sugar or honey
- half a can of non-diet soft drink
- 2-3 concentrated glucose tablets.
Unless you are within 10 to 15 minutes of your next meal or snack, follow up with extra carbohydrates such as plain biscuits, fruit or milk. Taking this extra carbohydrate will prevent a second drop in your blood glucose level.
HYPERGLYCAEMIA
If you notice the return of any signs of hyperglycaemia, contact your doctor immediately. The risk of hyperglycaemia is increased in the following situations:
- uncontrolled diabetes
- illness, infection or stress
- taking less Jardiamet than prescribed
- taking certain other medicines
- too little exercise
- eating more carbohydrates than normal.
Things you must not do
Do not take Jardiamet to treat any other complaints unless your doctor tells you to.
Do not give your medicine to anyone else, even if they have the same condition as you.
Do not stop taking your medicine or lower the dosage without checking with your doctor.
Things to be careful of
Be careful driving or operating machinery until you know how Jardiamet affects you.
When taken with other anti-diabetic medicines, such as sulfonylurea or insulin, your risk of getting low blood sugar is higher.
This may cause dizziness, lightheadedness, tiredness, drowsiness in some people. Low blood glucose levels may also slow your reaction time and affect your ability to drive or operate machinery.
If you have any of these symptoms, do not drive, operate machinery or do anything else that could be dangerous.
Be careful when doing any of the following things, which may increase the risk of your blood glucose becoming too low:
- drinking alcohol
- not eating enough
- doing unexpected or vigorous exercise.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking Jardiamet.
This medicine helps most people with type 2 diabetes mellitus, but it may have unwanted side effects in a few people.
All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical attention if you get some of the side effects.
Do not be alarmed by the following lists of side effects. You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
If you take Jardiamet, you may be at a greater risk of genital infections and urinary tract infections.
Tell your doctor or pharmacist if you notice any of the following and they worry you:
- genital burning, redness, pain and discharge which may be signs of a genital yeast infection
- passing more urine than normal
- itching
- loss in appetite
- loss of taste
- thirst
- diarrhoea or stomach ache
- feeling sick (nausea), vomiting
- constipation.
The above list includes the more common side effects of your medicine. They are usually mild and short-lived.
Tell your doctor as soon as possible if you notice any of the following:
- burning sensation when passing urine
- urine that appears cloudy
- pain in the pelvis, or mid-back pain
- straining or pain when passing urine
- unusual thirst
- light-headedness, or dizziness upon standing
- fainting or loss of consciousness.
The above list includes serious side effects that may require medical attention. Serious side effects are rare.
Tell your doctor immediately if you notice any of the symptoms of low blood sugar such as:
- sweating
- weakness
- hunger
- dizziness
- trembling
- headache
- flushing or paleness
- numbness
- a fast pounding heartbeat.
Low blood sugar may occur in patients who already take another medication to treat diabetes, such as a sulfonylurea or insulin. The dose of your sulfonylurea or insulin medicine may need to be reduced while taking Jardiamet.
Tell your doctor immediately if you experience pain or tenderness, itching, swelling in the genital or back passage area, fever or are generally feeling unwell. These may be symptoms of a serious and life-threatening infection called Fournier's gangrene. Your doctor may tell you to stop taking Jardiamet.
Tell your doctor immediately if you experience swelling of the penis that makes it difficult to pull back the skin around the tip of the penis (uncircumcised men).
Tell your doctor immediately or go to Emergency if you notice any of the following:
- sudden onset of hives, itching or skin rash
- swelling of the face, lips or tongue which may lead to difficulty swallowing or breathing.
Tell your doctor immediately or go to Emergency if you notice any of the symptoms of diabetic ketoacidosis such as:
- rapid weight loss
- feeling sick or being sick
- stomach pain
- excessive thirst
- fast and deep breathing
- confusion
- unusual sleepiness or tiredness
- a sweet smell to your breath, a sweet or metallic taste in your mouth or a different odour to your urine or sweat.
In rare cases, empagliflozin, one of the active substances in Jardiamet can cause a serious side effect called diabetic ketoacidosis.
Stop taking Jardiamet if you get any of the following symptoms of lactic acidosis and go to Emergency immediately:
- feeling cold (especially in your arms and legs)
- feeling very weak, tired
- feeling light-headed, dizzy
- severe nausea or vomiting
- feeling uncomfortable
- muscle pain
- drowsiness
- abdominal pain
- unexplained weight loss
- irregular heartbeat
- rapid or difficult breathing.
In rare cases, metformin, one of the active substances in Jardiamet can cause a serious side effect called lactic acidosis.
This is a medical emergency that can cause death. It is caused by build-up of lactic acid in your blood.
The above list includes very serious side effects. You may need urgent medical attention or hospitalisation. These side effects are very rare.
Tell your doctor or pharmacist if you notice anything that is making you feel unwell.
Other side effects not listed above may also occur in some people.
Some of these side effects can only be found when your doctor does tests from time to time to check your progress.
After using Jardiamet
Storage
Keep your tablets in the pack until it is time to take them. If you take the tablets out of the pack they may not keep well.
Keep your tablets in a cool dry place where the temperature stays below 30°C.
Do not store Jardiamet or any other medicine in the bathroom or near a sink. Do not leave it on a window sill or in the car. Heat and dampness can destroy some medicines.
Keep it where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking this medicine or the expiry date has passed, ask your pharmacist what to do with any medicine that is left over.
Product description
What it looks like
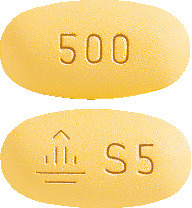
- Jardiamet 5 mg/500 mg tablets are orange yellow, oval, biconvex film-coated tablets. One side is debossed with the Boehringer Ingelheim company symbol and "S5" the other side is debossed with "500".
- Jardiamet 5 mg/850 mg tablets are yellowish white, oval, biconvex film-coated tablets. One side is debossed with Boehringer Ingelheim company symbol and "S5", the other side is debossed with "850".*
- Jardiamet 5 mg/1000 mg tablets are brownish yellow, oval, biconvex film-coated tablets. One side is debossed with Boehringer Ingelheim company symbol and "S5", the other side is debossed with "1000".
- Jardiamet 12.5 mg/500 mg tablets are pale brownish purple, oval, biconvex film-coated tablets. One side is debossed with Boehringer Ingelheim company symbol and "S12", the other side is debossed with "500".
- Jardiamet 12.5 mg/850 mg tablets are pinkish white, oval, biconvex film-coated tablets. One side is debossed with Boehringer Ingelheim company symbol and "S12", the other side is debossed with "850".*
- Jardiamet 12.5 mg/1000 mg tablets are dark brownish purple, oval, biconvex film-coated tablets. One side is debossed with Boehringer Ingelheim company symbol and "S12", the other side is debossed with "1000".
Jardiamet is available in blister packs containing 14 (sample) and 60 tablets.
* Not distributed in Australia.
Ingredients
Each Jardiamet 5 mg/500 mg tablet contains 5 mg empagliflozin and 500 mg metformin hydrochloride.
Each Jardiamet 5 mg/850 mg tablet contains 5 mg empagliflozin and 850 mg metformin hydrochloride.
Each Jardiamet 5 mg/1000 mg tablet contains 5 mg empagliflozin and 1000 mg metformin hydrochloride.
Each Jardiamet 12.5 mg/500 mg tablet contains 12.5 mg empagliflozin and 500 mg metformin hydrochloride.
Each Jardiamet 12.5 mg/850 mg tablet contains 12.5 mg empagliflozin and 850 mg metformin hydrochloride.
Each Jardiamet 12.5 mg/1000 mg tablet contains 12.5 mg empagliflozin and 1000 mg metformin hydrochloride.
Inactive ingredients:
- copovidone
- maize starch
- colloidal anhydrous silica
- magnesium stearate.
Tablet coating:
- hypromellose
- titanium dioxide
- macrogol 400
- purified talc
- iron oxide yellow (5 mg/500 mg, 5 mg/850 mg, 5 mg/1000 mg tablets)
- iron oxide black (12.5 mg/500 mg, 12.5 mg/850 mg, 12.5 mg/1000 mg tablets)
- iron oxide red (12.5 mg/500 mg, 12.5 mg/850 mg, 12.5 mg/1000 mg tablets).
This medicine does not contain lactose, sucrose, gluten, tartrazine or any other azo dyes.
Supplier
Jardiamet is supplied in Australia by:
Boehringer Ingelheim Pty Limited
ABN 52 000 452 308
Sydney NSW
www.boehringer-ingelheim.com.au
This Consumer Medicine Information was updated in September 2022.
® Jardiamet is a registered trade mark of Boehringer Ingelheim.
© Boehringer Ingelheim Pty Limited 2022.
Australian Registration Numbers
Jardiamet 5 mg/500 mg
(AUST R 229815)
Jardiamet 5 mg/850 mg
(AUST R 229816)
Jardiamet 5 mg/1000 mg
(AUST R 229817)
Jardiamet 12.5 mg/500 mg
(AUST R 229818)
Jardiamet 12.5 mg/850 mg
(AUST R 229819)
Jardiamet 12.5 mg/1000 mg
(AUST R 229820)
Published by MIMS November 2022