What is in this leaflet
When you must not use Kliogest®
What Kliogest® is used for
Before you take Kliogest®
How to take Kliogest®
While you are taking Kliogest®
Side effects
Storage
Product Description
Further information
User instructions
This leaflet answers some common questions about Kliogest®, the menopause (the ‘change of life’), and hormone therapy. It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you using Kliogest® against the benefits they expect it will have for you.
Ask your doctor or pharmacist if you have any concerns about using this medicine.
Keep this leaflet with the medicine.
You may need to read it again.
Kliogest® is available only by prescription at pharmacies.
When you must not use Kliogest®
Do not use Kliogest® or other estrogens, with or without a progestagen to prevent heart attacks, stroke or dementia.
A study called the Women’s Health Initiative indicated increased risk of heart attack, stroke, breast cancer, and blood clots in the legs or lungs in women receiving treatment with a product containing conjugated estrogens 0.625 mg and the progestagen medroxyprogesterone acetate (MPA). The researchers stopped the study after 5 years when it was determined the risks were greater than the benefits in this group. The Women’s Health Initiative Memory Study indicated increased risk of dementia in women aged 65-79 years taking conjugated estrogens and MPA. There are no comparable data currently available for other doses of conjugated estrogens and MPA or other combinations of estrogens and progestagens. Therefore, you should assume the risks will be similar for other medicines containing estrogen and progestagen combinations.
Talk regularly with your doctor about whether you still need treatment with Kliogest®.
Treatment with estrogens, with or without progestagens should be used at the lowest effective dose and for the shortest period of time.
What Kliogest® is used for
Kliogest® is a type of treatment called hormone replacement therapy (HRT). Each tablet contains two hormones, an estrogen (estradiol) and a progestagen (norethisterone acetate).
Kliogest® is used for:
Relief of symptoms occurring after menopause
During the menopause, the amount of the estrogen produced by a woman’s body drops. This can cause symptoms such as hot face, neck and chest (‘hot flushes’). Kliogest® alleviates these symptoms after menopause. You will only be prescribed Kliogest® if your symptoms seriously hinder your daily life.
Prevention of loss of bone mineral density
After the menopause some women may develop fragile bones (osteoporosis). You should discuss all available options with your doctor.
If you are at an increased risk of fractures due to osteoporosis and other medicines are not suitable for you, you can use Kliogest® to prevent loss of bone mineral density after menopause.
Kliogest® is prescribed for women who have not had their womb removed, and whose periods stopped more than a year ago.
How it works
The menopause, a woman's last menstrual period, usually occurs between the ages of 45 and 55 years. During this natural life transition, a woman slowly stops producing two female hormones called estrogen and progesterone. Menstrual periods become less frequent until they finally stop.
The falling hormone levels may cause you to experience uncomfortable symptoms such as hot flushes or night sweats.
Kliogest® contains two hormones, an estrogen (estradiol) and a progestagen (norethisterone acetate). The estradiol in Kliogest® is identical to the estradiol produced in the ovaries of women, and is classified as a natural estrogen. Norethisterone acetate is a synthetic progestagen which acts in a similar manner as progesterone, another important female sex hormone.
The estrogen in Kliogest® relieves the symptoms caused by a lack of estrogen. The progestagen protects the lining of your womb from overstimulation by estrogen.
Your doctor may have prescribed Kliogest® for another reason.
Ask your doctor if you have any questions about why Kliogest® has been prescribed for you.
Before you take Kliogest®
When you must not take it
Do not take Kliogest® if any of the following applies to you. Talk to your doctor before taking Kliogest® if you are not sure about any of the points below.
Do not take Kliogest®:
- if you have, have had or suspect having breast cancer
- if you have, have had or suspect having cancer of the womb lining (endometrial cancer), or any other estrogen dependent cancer
- if you have any unexplained vaginal bleeding
- if you have excessive thickening of the womb lining (endometrial hyperplasia) that is not being treated
- if you have or have ever had a blood clot in a vein (venous thromboembolism), such as in the legs (e.g. deep vein thrombosis), or the lungs (pulmonary embolism)
- if you have a blood clotting disorder (such as protein C, protein S or antithrombin deficiency)
- if you have or previously have had a disease caused by blood clots in the arteries, such as a heart attack, stroke or angina
- if you have or have ever had a liver disease and your blood test results have not returned to normal
- if you have a rare blood problem called ‘porphyria’ which is passed down in families (inherited)
- if you are allergic (hypersensitive) to estradiol, norethisterone acetate or any of the other ingredients of Kliogest® (listed in the Ingredients section of this leaflet)
- you are pregnant or suspect you may be pregnant, or you are breast-feeding
- it is after the expiry date (‘Expiry’) printed on the pack
- the packaging is torn or shows signs of tampering.
Before you start to take it
Medical history and regular check-ups
The use of HRT carries risks which need to be considered when deciding whether to start taking it or whether to carry on taking it.
Talk to your doctor if you have a premature menopause.
The experience in treating women with a premature menopause (due to ovarian failure or surgery) is limited. If you have a premature menopause the risks of using HRT may be different.
Before you start (or restart) HRT, your doctor will ask about your own and your family´s medical history. Your doctor may decide to perform a physical examination. This may include an examination of your breasts and/or an internal examination, if necessary.
Once you have started on Kliogest® you should see your doctor for regular check-ups (at least once a year).
At these check-ups, discuss with your doctor the benefits and risks of continuing with Kliogest®.
Go for regular breast screening as recommended by your doctor.
The experience of treating women older than 65 years’ is limited.
Warnings and precautions
Tell your doctor if you have ever had any of the following problems, before you start the treatment, as these may return or become worse during treatment with Kliogest®.
If so, you should see your doctor more often for check-ups:
- fibroids inside your womb
- growth of the womb lining outside your womb (endometriosis) or a history of excessive growth of the womb lining (endometrial hyperplasia)
- increased risk of developing blood clots (see Blood clots in a vein (venous thromboembolism))
- increased risk of getting an estrogen-sensitive cancer (such as having a mother, sister or grandmother who has had breast cancer)
- high blood pressure
- a liver disorder, such as a benign liver tumour
- diabetes
- gallstones
- migraine or severe headaches
- a disease of the immune system that affects many organs of the body (systemic lupus erythematosus, SLE)
- epilepsy
- asthma
- a disease affecting the eardrum and hearing (otosclerosis)
- a very high level of fat in your blood (triglycerides)
- fluid retention due to cardiac or kidney problems
- a condition where your thyroid gland fails to produce enough thyroid hormone (hypothyroidism) and you are treated with thyroid hormone replacement therapy
- a hereditary condition causing recurrent episodes of severe swelling (hereditary angioedema) or if you have had episodes of rapid swelling of the hands, face, feet, lips, eyes, tongue, throat (airway blockage) or digestive tract
- lactose intolerance.
Stop taking Kliogest® and see a doctor immediately
Stop taking Kliogest® and see a doctor immediately if you notice any of the following when taking HRT:
- any of the conditions mentioned in the When you must not take it section
- yellowing of your skin or the whites of your eyes (jaundice). These may be signs of a liver disease.
- a large rise in your blood pressure (symptoms may be headache, tiredness, dizziness)
- migraine-like headaches which happen for the first time
- if you become pregnant
- if you notice signs of a blood clot, such as:
- painful swelling and redness of the legs
- sudden chest pain
- difficulty in breathing.
For more information, see Blood clots in a vein (venous thromboembolism).
Note: Kliogest® is not a contraceptive. If it is less than 12 months since your last menstrual period or you are under 50 years old, you may still need to use additional contraception to prevent pregnancy. Speak to your doctor for advice.
HRT and cancer
Excessive thickening of the lining of the womb (endometrial hyperplasia) and cancer of the lining of the womb (endometrial cancer)
Taking estrogen-only HRT will increase the risk of excessive thickening of the lining of the womb (endometrial hyperplasia) and cancer of the womb lining (endometrial cancer).
The progestagen in Kliogest® reduces this extra risk.
Compare
In women who still have a womb and who are not taking HRT, on average, 5 in 1,000 will be diagnosed with endometrial cancer between the ages of 50 and 65.
For women aged 50 to 65 who still have a womb and who take estrogen-only HRT, between 10 and 60 women in 1,000 will be diagnosed with endometrial cancer (i.e. between 5 and 55 extra cases), depending on the dose and for how long it is taken.
Irregular bleeding
You may have irregular bleeding or drops of blood (spotting) during the first 3-6 months of taking Kliogest®.
However, see your doctor as soon as possible if the irregular bleeding:
- carries on for more than the first 6 months
- starts after you have been taking Kliogest® for more than 6 months
- carries on after you have stopped taking Kliogest®.
Breast cancer
Evidence shows that taking combined estrogen-progestagen or estrogen-only hormone replacement therapy (HRT) increases the risk of breast cancer. The extra risk depends on how long you use HRT. The additional risk becomes clear within 3 years of use. After stopping HRT the extra risk will decrease with time, but the risk may persist for 10 years or more if you have used HRT for more than 5 years.
Compare
Women aged 50 to 54 who are not taking HRT, on average, 13 to 17 in 1,000 will be diagnosed with breast cancer over a 5-year period. For women aged 50 who start taking estrogen-only HRT for 5 years, there will be 16-17 cases in 1,000 users (i.e. an extra 0 to 3 cases).
For women aged 50 who start taking estrogen-progestagen HRT for 5 years, there will be 21 cases in 1,000 users (i.e. an extra 4 to 8 cases).
Women aged 50 to 59 who are not taking HRT, on average, 27 in 1,000 will be diagnosed with breast cancer over a 10-year period.
For women aged 50 who start taking estrogen-only HRT for 10 years, there will be 34 cases in 1,000 users (i.e. an extra 7 cases).
For women aged 50 who start taking estrogen-progestagen HRT for 10 years, there will be 48 cases in 1,000 users (i.e. an extra 21 cases).
Regularly check your breasts. See your doctor if you notice any changes such as:
- dimpling of the skin
- changes in the nipple
- any lumps you can see or feel.
Additionally, you are advised to join mammography screening programs when offered to you. For mammogram screening, it is important that you inform the nurse/healthcare professional who is actually taking the x-ray that you use HRT, as this medication may increase the density of your breasts which may affect the outcome of the mammogram. Where the density of the breast is increased, mammography may not detect all lumps.
Ovarian cancer
Ovarian cancer is rare - much rarer than breast cancer. The use of estrogen-only or combined estrogen-progestagen HRT has been associated with a slightly increased risk of ovarian cancer. The risk of ovarian cancer varies with age. For example, in women aged 50 to 54 who are not taking HRT, about 2 women in 2,000 will be diagnosed with ovarian cancer over a 5-year period. For women who have been taking HRT for 5 years, there will be about 3 cases per 2,000 users (i.e. about 1 extra case).
Effect of HRT on heart and circulation
Blood clots in a vein (venous thromboembolism)
The risk of blood clots in the veins is about 1.3 to 3 times higher in HRT users than in non-users, especially during the first year of taking it.
Blood clots can be serious, and if one travels to the lungs, it can cause chest pain, breathlessness, fainting or even death.
You are more likely to get a blood clot in your veins as you get older and if any of the following applies to you. Inform your doctor if any of these situations applies to you:
- you are unable to walk for a long time because of major surgery, injury or illness (see also If you need to have surgery, below)
- you are seriously overweight (BMI >30 kg/m2)
- you have any blood clotting problem that needs long-term treatment with a medicine used to prevent blood clots
- if any of your close relatives has ever had a blood clot in the leg, lung or another organ
- you have systemic lupus erythematosus (SLE)
- you have cancer.
For signs of a blood clot, see Stop taking Kliogest® and see a doctor immediately, above.
Compare
Looking at women in their 50s who are not taking HRT, on average, over a 5-year period, 4 to 7 in 1,000 would be expected to get a blood clot in a vein.
For women in their 50s who have been taking estrogen-progestagen HRT for over 5 years, there will be 9 to 12 cases in 1,000 users (i.e. an extra 5 cases).
Heart disease (heart attack)
There is no evidence that HRT will prevent a heart attack. Women over the age of 60 years who use estrogen-progestagen HRT are slightly more likely to develop heart disease than those not taking any HRT.
Stroke
The risk of getting stroke is about 1.5 times higher in HRT users than in non-users. The number of extra cases of stroke due to use of HRT will increase with age.
Compare
Looking at women in their 50s who are not taking HRT, on average, 8 in 1,000 would be expected to have a stroke over a 5-year period.
For women in their 50s who are taking HRT, there will be 11 cases in 1,000 users over 5 years (i.e. an extra 3 cases).
Other conditions
HRT will not prevent memory loss. There is some evidence of a higher risk of memory loss in women who start using HRT after the age of 65. Speak to your doctor for advice.
Tell your doctor or the laboratory staff that you are taking Kliogest® if you need a blood test.
This is because this medicine can affect the results of some tests.
Taking other medicines
Tell your doctor if you are taking or plan to take other medicines, including:
- other estrogen medicines
- medicines to help you sleep, including barbiturates
- medicines for epilepsy e.g. phenytoin, carbamazepine, lamotrigine
- some antibiotics and other anti-infective medicines e.g. rifampicin, rifabutin, nevirapine, efavirenz, ritonavir, nelfinavir, ketoconazole
- medicines for hepatitis C infections (such as telaprevir)
- St. John’s Wort – used to treat depression
- antihistamines – used to treat allergies
- anticoagulants and antifibrinolytics – used to manage blood clotting processes
- antidiabetic drugs – used to treat diabetes
- medicines which decrease serum folate
- thyroid hormones – used to treat malfunction of the thyroid gland
- corticosteroids – used to treat inflammatory conditions.
Or other specific medicines including:
- imipramine
- pethidine
- cyclosporin.
Tell your doctor or pharmacist if you are taking any other medicines, including any that you buy without a prescription from your pharmacy, supermarket or health food shop.
The effect of Kliogest® can be reduced by other medicines, and may affect your vaginal bleeding pattern.
How to take Kliogest®
How to take it
Read carefully the instructions included in this leaflet, in order to correctly use the calendar pack.
Your doctor will tell you when to start taking the tablets. Kliogest® treatment should usually start a year after your periods stop.
If you are not already using HRT, you can start Kliogest® at a convenient time for you. If you are already using a different type of HRT, your doctor can advise you when to switch to Kliogest®.
Take one tablet a day, preferably at the same time each day, until all 28 tablets have been taken. Swallow each tablet with a glass of water. When you have finished each pack, start the next pack immediately.
How long to take it
HRT should be prescribed at the lowest effective dose and for the shortest duration necessary (see Before you start to take it). Your doctor can advise you how long you may need to take Kliogest®.
The continuation of the treatment should be re-evaluated annually.
If you forget to take it
You can always see if you have taken your tablet by looking at the day on the calendar pack.
If you forget to take a tablet at the usual time, take it as soon as you remember. If it is almost time for your next dose, throw away the tablet you missed and take your next dose when you are meant to.
You may have vaginal bleeding or spotting if you forget to take your tablets.
If you take too much (overdose)
If you take more tablets than you have been prescribed, contact your doctor for advice as soon as possible.
Overdose may cause nausea and vomiting.
If you need to have surgery
Tell the surgeon that you are taking Kliogest® if you are going to have surgery.
You may need to stop taking Kliogest® about 4 to 6 weeks before the operation to reduce the risk of a blood clot (see Blood clots in a vein (venous thromboembolism)).
Ask your doctor when you can start taking Kliogest® again.
While you are taking Kliogest®
You can expect your symptoms to improve within a few weeks of starting Kliogest®. However you will not be aware of the effect of Kliogest® on your bones.
Kliogest® can be stopped at any time. You should discuss this with your doctor.
You should include foods that are good sources of calcium and Vitamin D in your daily diet, and exercise regularly. Calcium, Vitamin D and exercise help prevent thinning of the bones. Your doctor can advise you on which foods and type of exercise are best for you.
Stop taking Kliogest® and contact your doctor if you become pregnant.
Kliogest® is for use in postmenopausal women only.
Do not take Kliogest® if you are breast-feeding.
At your routine check-up, your doctor may reassess your continued need for Kliogest®. Your doctor may consider alternative HRT treatments if troublesome symptoms remain.
If you have any concerns about taking Kliogest®, ask your doctor or pharmacist. If your doctor tells you to stop taking Kliogest®, return any unused medicine to your pharmacist.
Things you must not do
This medicine is for you only. Do not give it to someone else even if they seem to have the same symptoms as you.
Do not take Kliogest® to treat any other complaints unless your doctor tells you to.
Do not change the way you take Kliogest®, or lower the dosage, without checking with your doctor.
Side effects
All medicines can have side effects. Sometimes they are serious, most of the time they are not.
Tell your doctor or pharmacist if you experience any side effects while you are taking Kliogest® (whether or not they are mentioned below).
You may need medical treatment if you experience some of the side effects.
When you start taking Kliogest® your body has to adjust to new hormone levels. You may experience the following side effects:
- breast tenderness, pain or enlargement
- irregular periods or excessive bleeding during your periods
- period pain
- abdominal (stomach) pain, indigestion, feeling sick (nausea), vomiting, diarrhoea, bloating, flatulence
- changes in sexual appetite, problems getting to sleep, nervousness, anxiety
- swelling due to fluid retention (oedema)
- headache, migraine, dizziness
- skin rash or itching, skin reactions, acne, changes in hair growth, hair loss
- inflammation of a vein
- fungal infection of the vagina (thrush), vaginal inflammation or itching
- asthma
- gallbladder problems, gallstones
- leg cramps
- back pain
- weight change
- visual disturbances, dry eyes, tear film composition changes
- aggravation, occurrence or recurrence of uterine fibroids (benign tumours)
- blood clots
- Kliogest® does not treat your symptoms effectively.
These side effects are usually temporary and disappear.
Tell your doctor if:
- you think you may be suffering from depression
- you are not feeling well or find any side effect too uncomfortable or unacceptable
- any side effect becomes worse
- vaginal bleeding or spotting suddenly becomes heavier.
Tell your doctor immediately if any of the following conditions occur (because you may be told to stop taking Kliogest®):
- severe pain or swelling in your legs
- yellow colouring of the skin and eyes (jaundice)
- migraine or sudden severe headache, and you have not previously had migraines
- problems with your eyesight which develop suddenly
- marked rise in blood pressure
- you can see or feel a lump in your breast, or you notice dimpling of the skin or changes in the nipple
- you know or suspect you are pregnant.
Tell your doctor immediately or go to Accident and Emergency at your nearest hospital if you notice any of the following:
- skin rashes over a large part of the body
- shortness of breath, wheezing
- swelling of the face, lips or tongue
- fast pulse
- sweating.
This list includes very serious side effects. You may need urgent medical attention or hospitalisation. These side effects are very rare.
Effects on your heart or circulation
Heart disease
Talk to your doctor to see if you should be taking HRT if you have ever had heart disease.
HRT is not recommended for women who have heart disease, or have had heart disease recently.
HRT will not help to prevent heart disease.
Studies with one type of HRT (containing conjugated estrogen plus the progestagen MPA) have shown that women may be slightly more likely to get heart disease during the first year of taking the medication. For other types of HRT, the risk is likely to be similar, although this is not yet certain.
See a doctor as soon as possible and do not take any more HRT until your doctor says you can if you get:
- a pain in your chest that spreads to your arm or neck.
This pain could be a sign of heart disease.
Stroke
Recent research suggests that HRT slightly increases the risk of having a stroke. Other things that can increase the risk of stroke include:
- getting older
- high blood pressure
- smoking
- drinking too much alcohol
- an irregular heartbeat.
Talk to your doctor to see if you should take HRT if you are worried about any of these things, or if you have had a stroke in the past.
See a doctor as soon as possible and do not take any more HRT until your doctor says you can if you get:
- unexplained migraine-type headaches, with or without disturbed vision. These headaches may be an early warning sign of a stroke.
Blood clots
HRT may increase the risk of blood clots in the veins (also called deep vein thrombosis, or DVT), especially during the first year of taking it.
These blood clots are not always serious, but if one travels to the lungs, it can cause chest pain, breathlessness, collapse or even death. This condition is called pulmonary embolism, or PE. DVT and PE are examples of a condition called venous thromboembolism, or VTE.
You are more likely to get a blood clot if:
- you are seriously overweight
- you have had a blood clot before
- any of your close family have had blood clots
- you have had one or more miscarriages
- you have any blood clotting problem that needs treatment with a medicine such as warfarin
- you are off your feet for a long time because of major surgery, injury or illness
- you have a rare condition called systemic lupus erythematosus (SLE – an autoimmune disease).
If any of these things apply to you, talk to your doctor to see if you should take HRT.
See a doctor as soon as possible and do not take any more HRT until your doctor says you can if you get:
- painful swelling in your leg
- sudden chest pain
- difficulty breathing.
These may be signs of a blood clot.
Effects on your risk of developing cancer
Breast cancer
Women who have breast cancer, or have had breast cancer in the past, should not take HRT.
Taking HRT slightly increases the risk of breast cancer; so does having a later menopause. The risk for a post-menopausal woman taking estrogen-only HRT for 5 years is about the same as for a woman of the same age who is still having periods over that time and not taking HRT. The risk for a woman who is taking estrogen plus progestagen HRT is higher than for estrogen-only HRT (but estrogen plus progestagen HRT is beneficial for the endometrium, see Endometrial cancer below).
For all kinds of HRT, the extra risk of breast cancer goes up the longer you take it, but returns to normal within about 5 years after stopping HRT.
Your risk of breast cancer is also higher if:
- you have a close relative (mother, sister or grandmother) who has had breast cancer
- you are seriously overweight.
Make an appointment to see your doctor as soon as possible if you notice any changes in your breasts, such as:
- dimpling of the skin
- changes in the nipple
- any lumps you can see or feel.
Endometrial cancer (cancer of the lining of the womb)
Taking estrogen-only HRT for a long time can increase the risk of cancer of the lining of the womb (the endometrium). Taking a progestagen as well as the estrogen helps to lower the extra risk.
If you still have your womb, your doctor may prescribe a progestagen as well as estrogen. If so, these may be prescribed separately, or as a combined HRT product.
If you have had your womb removed (a hysterectomy), your doctor will discuss with you whether you can safely take estrogen without a progestagen.
If you have had your womb removed because of endometriosis, any endometrium left in your body may be at risk. So your doctor may prescribe HRT that includes a progestagen as well as an estrogen.
Your product, Kliogest®, contains a progestagen.
If you get breakthrough bleeding or spotting, it’s usually nothing to worry about, especially during the first few months of taking HRT.
Make an appointment to see your doctor if the bleeding or spotting:
- carries on for more than the first few months
- starts after you’ve been on HRT for a while
- carries on even after you’ve stopped taking HRT.
It could be a sign that your endometrium has become thicker.
Ovarian cancer
Long-term (at least 5 - 10 years’) use of estrogen-only HRTs and estrogen plus progestagen HRTs has been associated with an increased risk of ovarian cancer in some epidemiological studies.
In addition to the possible side effects listed above, dementia has been reported with HRT.
Do not be alarmed by this list of possible side effects.
You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
Storage
Keep all medicines out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and label after ‘Expiry’.
The expiry date refers to the last day of that month.
Keep Kliogest® in a cool dry place where the temperature stays below 25°C. Do not put Kliogest® in the refrigerator.
Keep the calendar pack in the outer carton in order to protect from light.
Do not throw away any medicines down the sink or in your household rubbish. Ask your pharmacist how to throw away medicines you no longer use.
These measures will help to preserve the environment.
Product Description
What Kliogest® looks like
Kliogest® comes in a calendar dial pack. Each pack holds 28 tablets. Each Kliogest® tablet is white and round and marked ‘NOVO 281’ on one side.
Ingredients
Each tablet contains 2 mg estradiol (as hemihydrate) and 1 mg norethisterone acetate as the active ingredients. The tablets also contain lactose, maize starch, hyprolose, purified talc, magnesium stearate, hypromellose and triacetate. Kliogest® is gluten-free.
Manufacturer
Kliogest® is made in Denmark and supplied in Australia by:
Novo Nordisk Pharmaceuticals Pty. Ltd.
Level 10
118 Mount Street
North Sydney NSW 2060
This leaflet was prepared on 25 October 2021.
Australian Registration Number:
AUST R 183856
Kliogest® is a trademark owned by Novo Nordisk Health Care AG, Switzerland.
NovoCare® is a trademark owned by Novo Nordisk A/S.
© 2021
Novo Nordisk A/S
Further information
For further information call the NovoCare® Customer Care Centre on 1800 668 626.
Always check the following websites to ensure you are reading the most recent version of the Kliogest® consumer medicine information:
www.novonordisk.com.au
https://www.ebs.tga.gov.au/
User instructions
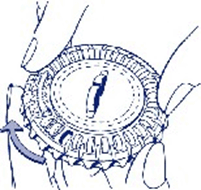
How to use the calendar dial pack
1. Set the day reminder
Turn the inner disc to set the day of the week opposite the little plastic tab.
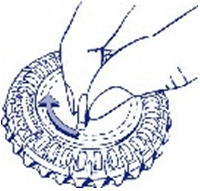
2. Take the first day’s tablet
Break the plastic tab and tip out the first tablet.
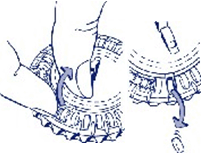
3. Move the dial every day
On the next day simply move the transparent dial clockwise one space as indicated by the arrow. Tip out the next tablet. Remember to take only one tablet once a day.
You can only turn the transparent dial after the tablet in the opening has been removed.