LESCOL XL®
| Consumer Medicine Information (CMI) summary |
The full CMI on the next page has more details. If you are worried about using this medicine, speak to your doctor or pharmacist.
| 1. Why am I using LESCOL XL? |
LESCOL XL contains the active ingredient fluvastatin. LESCOL XL is used to lower high cholesterol levels.
For more information, see Section 1. Why am I using LESCOL XL? in the full CMI.
| 2. What should I know before I take LESCOL XL? |
Do not take it if you have ever had an allergic reaction to LESCOL XL or any of the ingredients listed at the end of the CMI, or if you become pregnant while taking LESCOL XL.Talk to your doctor if you have any other medical conditions, take any other medicines, or are pregnant or plan to become pregnant or are breastfeeding.
For more information, see Section 2. What should I know before I take LESCOL XL? in the full CMI.
| 3. What if I am taking other medicines? |
Some medicines may interfere with LESCOL XL and affect how it works.
A list of these medicines is in Section 3. What if I am taking other medicines? in the full CMI.
| 4. How do I take LESCOL XL? |
- LESCOL XL prolonged released tablet is available in 80mg. To prevent complications in people with heart disease, the usual daily dose is 80 mg.
- Swallow each tablet whole with a drink of water. LESCOL XL must not be chewed, crushed, cut in half or split. You can take your tablet at any time of the day, with or without food.
- More instructions can be found in Section 4. How do I take LESCOL XL? in the full CMI.
| 5. What should I know while using LESCOL XL? |
| Things you should do |
|
| Things you should not do |
|
| Driving or using machines |
|
| Drinking alcohol |
|
| Looking after your medicine |
|
For more information, see Section 5. What should I know while using LESCOL XL? in the full CMI.
| 6. Are there any side effects? |
The most common side effects of LESCOL XL are headache, stomach discomfort or heartburn, nausea, and insomnia. Tell your doctor if you notice signs and symptoms of high levels of blood sugar such as an excessive thirst, high urine output, increased appetite with weight loss, and tiredness. Seek medical attention immediately if you experience any of the following, particularly if you also generally feel unwell: muscle aches, tenderness or weakness, with fever; swelling of the face, rash, difficulty breathing, coughing, yellowing of the skin and/or eyes and dark coloured urine, impaired brain function, easy bruising or bleeding. For more information, including what to do if you have any side effects, see Section 6. Are there any side effects? in the full CMI.
LESCOL XL®
Active ingredient(s): fluvastatin
| Consumer Medicine Information (CMI) |
This leaflet provides important information about using LESCOL XL. You should also speak to your doctor or pharmacist if you would like further information or if you have any concerns or questions about using LESCOL XL.
Where to find information in this leaflet:
1. Why am I using LESCOL XL?
2. What should I know before I take LESCOL XL?
3. What if I am taking other medicines?
4. How do I take LESCOL XL?
5. What should I know while using LESCOL XL?
6. Are there any side effects?
7. Product details
| 1. Why am I using LESCOL XL? |
LESCOL XL contains the active ingredient fluvastatin. LESCOL XL belongs to a group of medicines known as HMG-CoA reductase inhibitors (also known as 'statins').
LESCOL XL is used to lower high cholesterol levels.
The cholesterol-lowering effect slows the progression of atherosclerosis, which causes a thickening and hardening of the blood vessel walls due to cholesterol deposits and eventually blockage.
Everyone has cholesterol and triglycerides in their blood. They are a type of fat needed by the body for many things, such as building cells, making bile acids (which help to digest food) and making some hormones. However, having too much 'bad' cholesterol (LDL) in the blood can block the blood vessels that supply your heart and brain with blood, and can cause heart attack, angina and stroke. The 'good' cholesterol help remove the 'bad' cholesterol from the blood vessels. It is important to reduce the levels of ‘bad’ cholesterol and triglycerides in the blood if they are elevated and raise the levels of ‘good’ cholesterol in the blood.
Cholesterol is present in many foods and is also made by your body. If your body does not balance the amount of cholesterol it makes with the amount of cholesterol you eat, then your cholesterol becomes too high. High cholesterol is more likely to occur with certain diseases or if you have a family history of high cholesterol.
Lescol XL does not reduce the cholesterol that comes from fat in food. Therefore, you also need to follow a low-fat diet, control your weight and exercise regularly.
Lescol XL can also be used to help prevent further serious heart problems (e.g. heart attack) in people with heart disease who have already had heart catheterisation to open up the blood vessels in their heart.
| 2. What should I know before I take LESCOL XL? |
Warnings
Do not take LESCOL XL if:
- you are allergic to fluvastatin, or any of the ingredients listed at the end of this leaflet. Always check the ingredients to make sure you can take this medicine.
- you have active liver disease or unexplained persistent elevations in liver enzymes.
- you are pregnant, breast-feeding, or trying to become pregnant unless you are taking adequate contraceptive precautions.
- you have been prescribed any medicine containing fusidic acid.
Pregnancy and breastfeeding
Do not take LESCOL XL if you are pregnant or intend to become pregnant. Ask your doctor about effective methods of contraception.
If you become pregnant while taking LESCOL XL, stop taking it and tell your doctor immediately.
Do not breastfeed while you are taking LESCOL XL. Your baby may absorb this medicine and there is a possibility of harm to the baby.
Check with your doctor if you:
- have allergies to any other statins (e.g. atorvastatin, pravastatin, simvastatin). If you have an allergic reaction, you may have a skin rash, swelling of the face, lips or tongue, difficulty breathing or dizziness.
- have a personal or family history of hereditary muscular disorders.
- have a history of muscle problems from using other lipid-lowering agents.
- Have any of these medical conditions:
- liver or kidney problem.
- low thyroid hormone levels (hypothyroidism)
- a personal or family history of diabetes.
- severe metabolic, endocrine or electrolyte disorders such as decompensated diabetes and low blood potassium
- uncontrolled epilepsy.
- have very low blood pressure (signs may include dizziness, light-headedness).
It may not be safe to take LESCOL XL if you have any of these conditions. Your doctor will do a blood test before starting LESCOL XL.
- have any unexplained muscle pain, tenderness or weakness. These may be early signs of potentially severe muscle degradation.
- regularly drink large amounts of alcohol.
- have a serious infection, or about to have an operation.
During treatment, you may be at risk of developing certain side effects. It is important you understand these risks and how to monitor for them. See additional information under Section 6. Are there any side effects?
| 3. What if I am taking other medicines? |
Tell your doctor or pharmacist if you are taking any other medicines, including any medicines, vitamins or supplements that you buy without a prescription from your pharmacy, supermarket or health food shop.
Some medicines may interfere with LESCOL XL and affect how it works. These include:
- other cholesterol lowering medicines, including colestyramine, gemfibrozil and nicotinic acid.
- Ciclosporin, used to suppress the immune system.
- some antibiotics, including fusidic acid, rifampicin, erythromycin.
- Fluconazole, used to treat fungal infections.
- Phenytoin, used to treat epilepsy.
- Oral anticoagulants such as warfarin, used to reduce blood clotting.
- Glibenclamide, used to treat diabetes.
- Colchicine, used to treat gout and other inflammatory conditions.
Your doctor will consider if LESCOL XL should be taken together with any of these medicines or may wish to adjust the dose of the medicines. These medicines may affect the way LESCOL XL works.
Check with your doctor or pharmacist if you are not sure about what medicines, vitamins or supplements you are taking and if these affect LESCOL XL.
| 4. How do I take LESCOL XL? |
How much to take.
- Follow all directions given to you by your doctor and pharmacist carefully.
- LESCOL XL prolonged released tablet is available in 80mg.
- To prevent complications in people with heart disease, the usual daily dose is 80 mg.
- Swallow each tablet whole with a drink of water. LESCOL XL must not be chewed, crushed, cut in half or split.
- LESCOL XL helps to lower your 'bad' cholesterol but does not cure your condition. You must continue to take LESCOL XL to keep your cholesterol levels down.
When to take LESCOL XL
- LESCOL XL can be taken at any time of the day, with or without food.
- If you are also taking a medicine called colestyramine to help lower your cholesterol, take your dose of LESCOL XL at least 4 hours after taking the dose of colestyramine. These two medicines will interact with one another if they are taken too close together.
If you forget to take LESCOL XL
LESCOL XL should be taken regularly at the same time each day. If you forget to take a dose of LESCOL XL, take it as soon as you remember, as long as it is more than 4 hours before your next dose is due. Otherwise, wait until your next dose is due and take it as normal.
Do not take a double dose to make up for the dose you missed. This may increase the chance of you getting an unwanted side effect.
If you have trouble remembering when to take your medicine, ask your pharmacist for some tips.
If you take too much LESCOL XL
If you think that you have taken too much LESCOL XL, you may need urgent medical attention.
You should immediately:
- phone the Poisons Information Centre
(by calling 13 11 26), or - contact your doctor, or
- go to the Emergency Department at your nearest hospital.
You should do this even if there are no signs of discomfort or poisoning.
| 5. What should I know while using LESCOL XL? |
Things you should do.
Regularly have your blood cholesterol levels checked and any other blood tests done as your doctor says to make sure LESCOL XL is working and to prevent unwanted side effects.
Continue with your low-fat diet, control your weight and exercise regularly while you are taking LESCOL XL.
Call your doctor straight away if you:
- become pregnant while you are taking LESCOL XL.
Remind any doctor, dentist or pharmacist you visit that you are using LESCOL XL.
Things you should not do.
- Do not stop using LESCOL XL unless you have discussed it with your doctor.
Driving or using machines
Be careful before you drive or use any machines or tools until you know how LESCOL XL affects you.
LESCOL XL generally does not cause any problems with your ability to drive a car or operate machinery. However as with many other medicines, LESCOL XL may cause dizziness in some people.
Drinking alcohol
Tell your doctor if you regularly drink large amounts of alcohol.
Excessive alcohol consumption may not be safe in patients taking LESCOL XL.
Looking after your medicine
- Keep your tablets in the blister pack until it is time to take them.
Follow the instructions on the carton on how to take care of your medicine properly.
Store it in a cool dry place below 25°C and away from moisture, heat or sunlight; for example, do not store it:
- in the bathroom or near a sink, or
- in the car or on window sills.
Keep it where young children cannot reach it.
Getting rid of any unwanted medicine
If you no longer need to take this medicine or it is out of date, take it to any pharmacy for safe disposal.
Do not take this medicine after the expiry date.
| 6. Are there any side effects? |
All medicines can have side effects. If you do experience any side effects, most of them are minor and temporary. However, some side effects may need medical attention.
See the information below and, if you need to, ask your doctor or pharmacist if you have any further questions about side effects.
Less serious side effects
| Less serious side effects | What to do |
| Speak to your doctor if you have any of these less serious side effects and they worry you. |
Serious side effects
| Serious side effects | What to do |
Metabolism-related:
| Call your doctor straight away, or go straight to the Emergency Department at your nearest hospital if you notice any of these serious side effects. |
Tell your doctor or pharmacist if you notice anything else that may be making you feel unwell.
Other side effects not listed here may occur in some people.
Reporting side effects
After you have received medical advice for any side effects you experience, you can report side effects to the Therapeutic Goods Administration online at www.tga.gov.au/reporting-problems. By reporting side effects, you can help provide more information on the safety of this medicine.
Always make sure you speak to your doctor or pharmacist before you decide to stop taking any of your medicines.
| 7. Product details |
This medicine is only available with a doctor's prescription.
What LESCOL XL contains
| Active ingredient (main ingredient) | Fluvastatin sodium |
| Other ingredients (inactive ingredients) |
|
| Potential allergens | Lescol XL does not contain sucrose, gluten, tartrazine or any other azo dyes. |
Do not take this medicine if you are allergic to any of these ingredients.
What LESCOL XL looks like
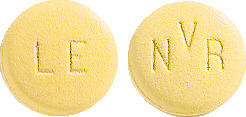
LESCOL XL 80 mg are yellow, round, slightly biconvex film-coated tablet, marked "LE" on one side and "NVR" on the other; packs of 28 (AUST R 82743).
Who distributes LESCOL XL
Novartis Pharmaceuticals Australia Pty Limited
54 Waterloo Road
Macquarie Park
NSW 2113
Australia
Telephone: 1 800 671 203
® = Registered Trademark
This leaflet was prepared in September 2023.
Internal document code:
les130923c based on PI les130923i
Published by MIMS November 2023