What is in this leaflet
This leaflet answers some of the common questions about Alfentanil injection.
It does not contain all the information. It does not take the place of talking to your doctor, anaesthetist or pharmacist.
All medicines have risks and benefits. Your pharmacist or doctor has weighed the risks of you being given Alfentanil injection against the benefits they expect it will have for you.
If you have any concerns about being given this medicine, ask your doctor, anaesthetist or pharmacist.
Keep this leaflet.
You may need to read it again.
What Alfentanil injection is used for
Alfentanil hydrochloride is a drug used to relieve pain and produce anaesthesia.
It can be used as a premedication before an operation, or with a general anaesthetic during an operation.
It belongs to a group of medicines called opioid analgesics.
It works by changing the messages that are sent to the brain about pain.
Your doctor will have explained why you are being treated with Alfentanil injection and told you what dose you will be given.
Follow all directions given to you by your doctor carefully. They may differ from the information contained in this leaflet.
Your doctor may prescribe this medicine for another use.
Ask your doctor if you want more information.
Alfentanil injection can be addictive, but when it is used only to relieve or prevent pain it is unlikely to become habit forming.
Before you are given Alfentanil injection
When you must not use it
Alfentanil injection should not be used for pain relief after surgery has taken place.
Alfentanil injection should not be used if you have an allergy or hypersensitivity to:
- alfentanil hydrochloride
- any ingredients listed at the end of this leaflet
- other opioid analgesics (pain killers) e.g. morphine or pethidine.
Symptoms of an allergic or hypersensitivity reaction may include:
- rash, itching or hives on the skin
- shortness of breath, wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body.
Alfentanil injection is not generally given to children under 12 years of age.
Alfentanil injection will only be used if the solution is clear, the package is undamaged and the use by (expiry) date marked on the pack has not passed.
Before you are given it
You must tell your doctor if you:
- are pregnant or planning to become pregnant. Your doctor will decide if you can take Alfentanil injection. It may affect your baby if it is given early in pregnancy or in the last weeks before your baby is due
- are breastfeeding or wish to breastfeed. Alfentanil injection may be excreted in breast milk. Breast- feeding is not advisable for 24 hours after Alfentanil injection has been given.
Tell your doctor if you have any of the following medical conditions:
- problems with your breathing such as severe asthma, severe bronchitis or emphysema
- a history of fits or head injury
- under-active thyroid
- myasthenia gravis (muscle weakness)
- heart problems
- liver or kidney problems
- are overweight or obese.
Tell your doctor if you take any medicine that slows down your reactions (CNS depressants), especially benzodiazepines or related drugs or have problems with alcohol.
It may not be safe for you to be given Alfentanil injection or you may be given a reduced dose if you have any of these conditions.
If you have not told your doctor about any of the above, tell them before you are given Alfentanil injection.
Taking other medicines
Tell your doctor if you are taking any other medicines, including medicines you can buy without a prescription from a pharmacy, supermarket or health food store.
Tell your doctor immediately and do not take Alfentanil injection if you are taking:
- medicines for depression called Monoamine Oxidase (MAO) Inhibitors. These medicines must not be taken in the 14 days before Alfentanil injection is given.
Also tell your doctor if you are taking:
- any anaesthetic agents such as propofol
- any medicine that slows down your reactions (CNS depressants) such as benzodiazepines or related drugs, sleeping pills, tranquillizers, medicines for mental disorders, alcohol, some illegal drugs.
If you receive a strong pain killer or other CNS depressant after receiving Alfentanil injection during surgery, the dose of the painkiller or other CNS depressants may need to be lowered to reduce the risk of potentially serious side effects such as breathing difficulties, with slow or shallow breathing, severe drowsiness and decreased awareness, coma and death.
- an antibiotic called erythromycin
- an antifungal called fluconazole, voriconazole, ketoconazole or itraconazole
- a medicine for the stomach called cimetidine
- an antiviral called ritonavir
- a heart medicine called diltiazem
- medicines for depression known as selective serotonin re-uptake inhibitors (SSRIs) or serotonin inhibitors (SNRIs).
Alfentanil injection can increase the effects of alcohol.
Tell your doctor about your consumption of alcohol and follow the doctor's advice.
If you have not told your doctor about any of these things, tell them before you are given Alfentanil injection.
These medicines may be affected by Alfentanil injection or may affect how well Alfentanil injection works. Your doctor can tell you what to do if you are taking any of these medicines.
Addiction
You can become addicted to Alfentanil injection even if you take it exactly as prescribed. Alfentanil injection may become habit forming causing mental and physical dependence. If abused it may become less able to reduce pain.
Dependence
As with all other opioid containing products, your body may become used to you taking Alfentanil injection. Taking it may result in physical dependence. Physical dependence means that you may experience withdrawal symptoms if you stop taking Alfentanil injection suddenly, so it is important to take it exactly as directed by your doctor.
Tolerance
Tolerance to Alfentanil injection may develop, which means that the effect of the medicine may decrease. If this happens, more may be needed to maintain the same effect.
Withdrawal
Continue taking your medicine for as long as your doctor tells you. If you stop having this medicine suddenly, your pain may worsen and you may experience some or all of the following withdrawal symptoms:
- nervousness, restlessness, agitation, trouble sleeping or anxiety
- body aches, weakness or stomach cramps
- loss of appetite, nausea, vomiting or diarrhoea
- increased heart rate, breathing rate or pupil size
- watery eyes, runny nose, chills or yawning
- increased sweating.
Alfentanil injection given to the mother during labour can cause breathing problems and signs of withdrawal in the newborn.
Taking Alfentanil injection
Alfentanil injection will be given to you by injection by a specially trained anaesthetist.
The injection is given into the vein (intravenous use).
Your doctor will decide how much Alfentanil injection you will need.
Elderly people may be given a smaller dose.
If you take too much (Overdose)
The doctor or nurse giving you Alfentanil injection will be experienced in its use, so it is extremely unlikely that you will be given too much.
In the unlikely event that an overdose occurs, your doctor or the anaesthetist will take the necessary actions. The symptoms of overdose could include:
- slow, unusual or difficult breathing
- drowsiness, dizziness or unconsciousness
- slow or weak heartbeat
- nausea or vomiting
- convulsions or fits
- muscle stiffness
- lowering of blood pressure
- lowering of heart rate.
If these symptoms occur, you may be administered another medicine (e.g. naloxone) to help reverse the effects.
If you think you or anybody else has been given too much Alfentanil injection, contact your doctor or nurse immediately or phone the Poisons Information Centre (by calling 13 11 26).
Side effects
Tell your doctor or nurse as soon as possible if you do not feel well after you have been given Alfentanil injection.
Alfentanil injection helps most people suffering severe pain, but it may have unwanted side-effects in a few people. All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical treatment if you get some of the side effects.
Ask your doctor, nurse or pharmacist to answer any questions you may have.
After you have been given Alfentanil injection you will probably feel light-headed, dizzy, sleepy and you may feel quite strange, especially if you are not lying down.
Tell your doctor or nurse if you notice any of the following side effects and they worry you:
- nausea and vomiting
- dizziness
- drowsiness or sleepiness
- injection site pain or pain during the procedure.
Tell your doctor or nurse as soon as possible if you have any of the following as you may need medical attention:
- feeling of extreme happiness (euphoric mood)
- visual disturbance such as blurred vision
- chills
- rash.
Tell your doctor or nurse immediately if you experience:
- breathing difficulties, which can last longer than its pain-killing effect.
- slow, fast or irregular heartbeat.
- tightening of the chest or heart attack
- low or high blood pressure
- muscle stiffness or involuntary muscle movements, including slow, stiff or jerking movements
- spasm of the larynx (voice box).
Alfentanil injection may affect your alertness and ability to drive. Therefore you should not drive or operate machinery until your doctor advised that you can.
Some people may get other side effects after being given Alfentanil injection.
Tell your doctor or nurse if you notice anything else that is making you feel unwell.
After being given Alfentanil injection
Storage
Alfentanil injection should be kept in a cool dry place where the temperature stays below 25°C.
Alfentanil injection will be kept in a locked cupboard in the hospital pharmacy or operating theatre.
Alfentanil injection should not be used after the date (month and year) printed after "EXP". The anaesthetist will inspect Alfentanil injection before use to determine that it is still within its use by date.
Product is for single use. Once opened, any unused portion should be discarded.
Disposal
The hospital staff looking after you will dispose of any remaining Alfentanil injection appropriately.
Product description
What it looks like
Alfentanil injection comes in a clear glass ampoule containing a clear, colourless solution.
There are different presentations available:
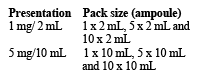
Not all presentations and pack sizes are marketed.
Ingredients
The active ingredient in Alfentanil injection is alfentanil hydrochloride, equivalent to 0.5 mg/mL of alfentanil.
Alfentanil injection also contains sodium chloride, sodium hydroxide (to adjust pH) and water for injections.
The 2 mL ampoule contains 1 mg of alfentanil.
The 10 mL ampoule contains 5 mg of alfentanil.
This medicine does not contain lactose, sucrose, gluten, tartrazine or other azo dyes.
Sponsor
Australian Sponsor
Medicianz Healthcare Pty Ltd
Unit 2, 6-7 Gilda Court
MULGRAVE VICTORIA 3170
Marketed and distributed by 
Medsurge Healthcare Pty Ltd
Tel: 1300 788 261
www.medsurge.com.au
Registration Numbers
AUST R 311910 (1 mg/2 mL)
AUST R 311911 (5 mg/10 mL)
This leaflet was prepared in September 2021.
