Mekinist®
| Consumer Medicine Information (CMI) summary |
The full CMI on the next page has more details. If you are worried about using this medicine, speak to your doctor or pharmacist.
| ▼ This medicine is new or being used differently. Please report side effects. See the full CMI for further details. |
| 1. Why am I using MEKINIST? |
MEKINIST contains the active ingredient trametinib. In adult patients MEKINIST is used to treat certain types of skin cancer (melanoma), thyroid cancers and lung cancers; also used to prevent the melanoma coming back if removed by surgery. In children 1 year and older, MEKINIST is used to treat brain tumours, following other treatments.
For more information, see Section 1. Why am I using MEKINIST? in the full CMI.
| 2. What should I know before I use MEKINIST? |
Do not use if you have ever had an allergic reaction to trametinib or any of the ingredients listed at the end of the CMI.
Talk to your doctor if you have any other medical conditions, take any other medicines, or are pregnant or plan to become pregnant or are breastfeeding. For more information, see Section 2. What should I know before I use MEKINIST? in the full CMI.
| 3. What if I am taking other medicines? |
Some medicines may interfere with MEKINIST and affect how it works. A list of these medicines is in Section 3. What if I am taking other medicines? in the full CMI.
| 4. How do I use MEKINIST? |
- MEKINIST should be taken on an empty stomach at least 1 hour BEFORE eating or 2 hours AFTER eating
- In adults, the usual total dose of MEKINIST is one 2 mg tablet once a day, taken at the same time each day
- MEKINIST tablet: Swallow the MEKINIST tablet whole with a full glass of water
- In children, dosage is based on body weight.
- MEKINIST powder is made into a solution and taken as a liquid oral solution only. Follow Instruction for Use in box.
More instructions can be found in Section 4. How do I use MEKINIST? in the full CMI.
| 5. What should I know while using MEKINIST? |
| Things you should do |
|
| Things you should not do |
|
| Driving or using machines |
|
| Drinking alcohol |
|
| Looking after your medicine |
|
For more information, see Section 5. What should I know while using MEKINIST? in the full CMI.
| 6. Are there any side effects? |
Common side effects include cough, headache, fever, feeling tired/sick, diarrhoea, vomiting, rash, dry mouth, itching, hair loss/thinning, swelling, flu-like symptoms, joint or muscle pain, blurry vision, mouth sores, nose bleeds, bruising, cracked/dry/red or irritated skin, weakness. Severe side effects include severe tummy pain, blood in poo or vomit, severe cramps, slow or fast heart rate, difficulty breathing, wheezing, swelling of face, hands, lips or eyes, vision loss, sense of flashing lights, sudden bleeding. For more information, including what to do if you have any side effects, see Section 6. Are there any side effects? in the full CMI.
| ▼ This medicine is subject to additional monitoring due to approval of an extension of indications. This will allow quick identification of new safety information. You can help by reporting any side effects you may get. You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems. |
Mekinist® (MEH-kih-nist)
Active ingredient(s): trametinib dimethyl sulfoxide
| Consumer Medicine Information (CMI) |
This leaflet provides important information about using MEKINIST. You should also speak to your doctor or pharmacist if you would like further information or if you have any concerns or questions about using MEKINIST.
Where to find information in this leaflet:
1. Why am I using MEKINIST?
2. What should I know before I use MEKINIST?
3. What if I am taking other medicines?
4. How do I use MEKINIST?
5. What should I know while using MEKINIST?
6. Are there any side effects?
7. Product details
| 1. Why am I using MEKINIST? |
MEKINIST contains the active ingredient trametinib.
MEKINIST belongs to a group of medicines called "selective MEK-inhibitors".
MEKINIST is used to:
- Treat adult patients with the following types of cancers that have spread to other parts of the body:
- skin cancers called melanoma
- thyroid cancers called anaplastic thyroid cancer (ATC)
- lung cancers called non-small cell lung cancer (NSCLC) - Prevent melanoma from coming back after the melanoma has been removed by surgery in adult patients.
- Treat children 1 year and older with a brain tumour (glioma), following other treatments.
These cancers have a change (mutation) in a gene called "BRAF" that may have caused the cancer to grow. MEKINIST targets proteins made from this changed gene and slows down or stops the growth of your cancer.
MEKINIST can be used by itself or in combination with another medicine called TAFINLAR (containing dabrafenib).
If you are taking these two medicines together, read the full TAFINLAR Consumer Medicine Information as well as this one carefully.
| 2. What should I know before I use MEKINIST? |
Monitoring
Before you take MEKINIST, your doctor will take tumour tissue samples to check whether MEKINIST is suitable for you.
Your doctor should check your heart before you start taking MEKINIST and during treatment.
Your doctor may arrange for you to have an eye examination before you take MEKINIST and while you are taking it.
Warnings
Do not use MEKINIST if:
- You are pregnant
- You are allergic to "trametinib", or any of the ingredients listed at the end of this leaflet (symptoms of an allergic reaction can include difficulty breathing, wheezing, swelling of the face, lips or tongue and rash.
- You are under 12 months of age. It is not known whether it is safe and effective in this younger group of patients.
Check with your doctor if you have:
- Heart problems
- Eye problems
- Bleeding problems
- Lung problems or difficulty breathing
- Skin problems such as an acne-like rash
- Liver problems
- Gut problems
Pregnancy and breastfeeding
- If you are pregnant or breast-feeding, think you may become pregnant or are planning to have a baby, ask your doctor, pharmacist, or health care provider for advice before taking this medicine.
- If you do become pregnant while you are taking MEKINIST, tell your doctor immediately.
- Your doctor will discuss with you the potential risk(s) of taking MEKINIST during pregnancy or breast-feeding. MEKINIST alone or in combination with TAFINLAR are not recommended during pregnancy or while breastfeeding and may harm your baby.
Birth control requirements
Women taking MEKINIST
If you are a woman who could become pregnant, you must use good birth control (contraception) while you are taking MEKINIST and for at least 16 weeks after you stop taking it.
If MEKINIST is taken in combination with TAFINLAR, non-hormonal contraception should also be used.
Birth control methods containing hormones (such as pills, injections, or patches) may not work as well while taking TAFINLAR.
Men taking MEKINIST
Male patients (including those who have had a vasectomy) with female partners who are or who may become pregnant should use condoms during sexual intercourse while on treatment and for at least 16 weeks after stopping MEKINIST.
During treatment, you may be at risk of developing certain side effects. It is important you understand these risks and how to monitor for them. See additional information under Section 6. Are there any side effects?
| 3. What if I am taking other medicines? |
Tell your doctor or pharmacist if you are taking any other medicines, including any medicines, vitamins, or supplements that you buy without a prescription from your pharmacy, supermarket, or health food shop.
Some medicines may interfere with MEKINIST and affect how it works.
Check with your doctor or pharmacist if you are not sure about what medicines, vitamins or supplements you are taking and if these affect MEKINIST.
| 4. How do I use MEKINIST? |
How much to take
Adults
- The usual dose of MEKINIST is one 2 mg tablet once a day.
- Follow the instructions provided and use MEKINIST until your doctor tells you to stop.
- Do not take more MEKINIST than your doctor has recommended.
Children (1 year and above)
- The daily dose is based on the body weight and will be advised by their doctor.
- Follow the instructions provided and give MEKINIST until their doctor tells you to stop.
- Do not give more MEKINIST than their doctor has recommended.
When to take MEKINIST TABLETS
- Take MEKINIST once a day. Take it at the same time each day.
How to take MEKINIST TABLETS
- Take MEKINIST on an empty stomach.
- Swallow the MEKINIST tablet whole with a full glass of water
- MEKINIST should be taken either at least:
- 1 hour BEFORE eating. After taking MEKINIST, wait at least one hour before eating
OR
- 2 hours AFTER eating. After eating, wait at least two hours before taking MEKINIST.
When to give MEKINIST FOR ORAL SOLUTION
- Give MEKINIST once a day. Give it at the same time each day.
How to give MEKINIST for ORAL SOLUTION
MEKINIST powder needs to be prepared into a solution before it is given. Your pharmacist will prepare the oral solution for you. If you are given a MEKINIST bottle containing powder, do not give MEKINIST and contact your pharmacist.
Follow the Instruction for Use enclosed in the box on how to give MEKINIST oral solution. Talk to your doctor or pharmacist if you are not sure.
- Give MEKINIST on an empty stomach.
- MEKINIST should be given either at least:
- 1 hour BEFORE eating. After giving MEKINIST, wait at least one hour before eating
OR
- 2 hours AFTER eating. After eating, wait at least two hours before giving MEKINIST.
If you forget to take MEKINIST
MEKINIST should be taken regularly at the same time each day. If the missed dose of MEKINIST is:
- Less than 12 hours late, take it as soon as you remember.
- More than 12 hours late, skip that dose and take your next dose at the usual time. Then go back to taking your medicine normally.
Do not take a double dose to make up for the dose you missed.
If you use too much MEKINIST
If you think that you have taken too much MEKINIST (or TAFINLAR if using them together), you may need urgent medical attention.
You should immediately:
- phone the Poisons Information Centre
(by calling 13 11 26), or - contact your doctor, or
- go to the Emergency Department at your nearest hospital.
You should do this even if there are no signs of discomfort or poisoning.
| 5. What should I know while using MEKINIST? |
Things you should do
- Use contraception for both men and women to avoid pregnancy while taking MEKINIST and do this for 16 weeks after stopping MEKINIST
- If taking MEKINIST and TAFINLAR together, non-hormonal contraception should also be used (e.g., condoms)
- If you become pregnant while taking MEKINIST, tell your doctor immediately
- Keep all your doctor's appointments so that your progress can be checked
- Have the blood tests your doctor tells you to have
- Have your eyes, liver, kidneys, blood pressure and heart checked regularly.
Call your doctor straight away if you:
- Get a high fever (Signs of a fever may include a temperature of 38°C or more, severe chills, shivering, thirst, or dehydration, feeling dizzy, faint, or as if you're going to be sick).
Things you should not do
- Do not stop using this medicine suddenly
- Do not take MEKINIST with food.
Additional tests
- Your doctor may do some blood tests such as checking blood cells (white blood cells, red blood cells and platelets), electrolytes (e.g., sodium and phosphorus) and sugar levels in your body from time to time to make sure MEKINIST is working and to prevent unwanted side effects. Your eyes, kidneys, liver, heart, blood pressure will also be checked regularly.
Driving or using machines
Be careful before you drive or use any machines or tools until you know how MEKINIST affects you.
Drinking alcohol
Tell your doctor if you drink alcohol.
Looking after your medicine
- Keep your tablets or the prepared solution in the bottle until it is time to take them.
- Tablets: Do not remove the desiccant (material that keeps the medicine dry)
- Keep your tablets and powder that has not been prepared in the refrigerator between 2°C and 8°C.
- Once powder has been prepared into a solution, keep below 25°C. Do not freeze.
- Once powder has been prepared into a solution, discard solution after a maximum of 35 days.
Whilst in use, the tablet bottle may be stored out of the refrigerator for a maximum of 30 days.
Follow the instructions on the carton on how to take care of your medicine properly.
Store it in a cool dry place away from moisture, heat, or sunlight; for example, do not store it:
- in the bathroom or near a sink, or
- in the car or on windowsills.
Keep it where young children cannot reach it.
Getting rid of any unwanted medicine
If you no longer need to use this medicine or it is out of date, take it to any pharmacy for safe disposal.
Do not use this medicine after the expiry date.
| 6. Are there any side effects? |
All medicines can have side effects. If you do experience any side effects, most of them are minor and temporary. However, some side effects may need medical attention.
See the information below and, if you need to, ask your doctor or pharmacist if you have any further questions about side effects.
Less serious side effects
| Less serious side effects | What to do |
| MEKINIST alone Hair problems
Skin problems
| Speak to your doctor if you have any of these less serious side effects and they worry you. If these side effects become severe, please tell your doctor, pharmacist, or healthcare provider. |
Serious side effects
| Serious side effects | What to do |
| MEKINIST alone Tummy problems
Signs of infection
| Call your doctor straight away or go straight to the Emergency Department at your nearest hospital if you notice any of these serious side effects. |
Tell your doctor or pharmacist if you notice anything else that may be making you feel unwell.
Other side effects not listed here may occur in some people.
Reporting side effects
After you have received medical advice for any side effects you experience, you can report side effects to the Therapeutic Goods Administration online at www.tga.gov.au/reporting-problems. By reporting side effects, you can help provide more information on the safety of this medicine.
Always make sure you speak to your doctor or pharmacist before you decide to stop taking any of your medicines.
| 7. Product details |
This medicine is only available with a doctor's prescription.
What MEKINIST contains
| Active ingredient (main ingredient) | Trametinib dimethyl sulfoxide |
| Other ingredients - tablets (inactive ingredients) | Mannitol (E421) Microcrystalline cellulose (E460(i)) Hypromellose (E464) Croscarmellose sodium Magnesium stearate (vegetable source) (E572) Sodium lauryl sulphate (E487) Colloidal anhydrous silica Titanium dioxide (E171) Polyethylene glycol 0.5 mg tablets only Iron oxide yellow CI 77492 (E172). 2 mg tablets only Polysorbate 80 (E433) Iron oxide red CI 77491 (E172) |
| Other ingredients - powder for oral solution (inactive ingredients) | Sucralose Citric acid monohydrate Dibasic sodium phosphate Sulfobutyl betadex sodium Potassium sorbate Methyl hydroxybenzoate Flavour strawberry |
| Potential allergens | MEKINIST tablets & powder do not contain lactose, gluten, tartrazine, or any other azo dyes. Powder: Contains sucrose |
Do not take this medicine if you are allergic to any of these ingredients.
What MEKINIST looks like
MEKINIST tablets are supplied in two strengths and are available in plastic bottles containing 30 tablets. The bottle has a child resistant closure and contains a desiccant. MEKINIST is also available as a powder (to be made into a solution) which is supplied in a glass bottle with a child resistant closure.
0.5 mg tablets
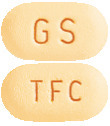
Yellow, modified oval, biconvex, film-coated tablets with 'GS' debossed on one face and 'TFC' on the opposite face (AUST R 205917).
2 mg tablets
Pink, round, biconvex, film-coated tablets with 'GS' debossed on one face and 'HMJ' on the opposite face (AUST R 205919).
4.7 mg powder for oral solution
White or almost white powder.
(AUST R 397190).
Who distributes MEKINIST:
MEKINIST is supplied in Australia by:
Novartis Pharmaceuticals Australia Pty Limited
ABN 18 004 244 160
54 Waterloo Road, Macquarie Park NSW 2113 Australia
Telephone 1 800 671 203
www.novartis.com.au
® Registered Trademark
This leaflet was prepared in December 2023.
Internal document code
(mek291123c based on PI mek291123i)
Published by MIMS January 2024