What is in this leaflet
This leaflet answers some common questions about Morphine Sulfate injection.
It does not contain all the available information.
It does not take the place of talking to your doctor or pharmacist.
The information in this leaflet was last updated on the date listed on the final page. More recent information on the medicine may be available.
You should ensure that you speak to your pharmacist or doctor to obtain the most up-to-date information on the medicine.
Those updates may contain important information about the medicine and its use of which you should be aware. All medicines have risks and benefits.
Your doctor has weighed the risks of you having Morphine Sulfate injection against the benefits they expect it will give you.
If you have any concerns about this medicine, ask your doctor or pharmacist.
Keep this leaflet.
You may need to read it again.
What Morphine Sulfate injection is used for
Morphine is a pain reliever that belongs to a group of medicines called opioid analgesics.
Morphine acts in the brain and spinal cord.
It is used most commonly for short-term relief of severe pain. It may also be used just before or during an operation to help the anaesthetic work better.
Ask your doctor if you have any questions about why this medicine has been prescribed for you.
Your doctor may have prescribed it for another purpose.
Morphine may produce physical dependency if used for a long time (i.e. more than two weeks). Physical dependency means you may experience unpleasant feelings if you stop morphine suddenly.
However, it is also important to keep your pain under control. Your doctor can advise you on how to manage this.
This medicine is only available with a doctor's prescription.
Before you use Morphine Sulfate injection
When you must not use Morphine Sulfate injection
Do not have Morphine Sulfate injection if you have ever had an allergic reaction after taking:
- morphine sulfate pentahydrate (the active ingredient in Morphine Sulfate injection)
- any of the other ingredients listed at the end of this leaflet
- any other similar medicines (such as medicines of the same class or with similar structure, as per PI).
Symptoms of an allergic reaction to morphine may include:
- shortness of breath, wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body
- rash, itching or hives on the skin.
You should not be given Morphine Sulfate injection if:
- you have severe bronchial asthma or any other lung or breathing problems
- you have shallow or difficulty breathing
- you are suffering from acute alcoholism
- you are undergoing treatment with monoamine oxidase (MAO) inhibitors (e.g. phenelzine, tranylcypromine, moclobemide or selegeline), or have stopped MAO inhibitor treatment during the last fourteen days
- you have an irregular heart beat (arrhythmia)
- you have severe liver problems
- severe central nervous system depression
- diabetic acidosis where there is danger of coma
- following biliary tract surgery or biliary colic
- obstruction of the gastrointestinal tract
- heart failure after lung disease
- you have a head injury, brain tumour or increased pressure in the head.
Morphine Sulfate injection must not be given to premature infants or during labour for delivery of premature infants.
Do not have Morphine Sulfate injection after the expiry date printed on the pack or if the packaging is torn or shows signs of tampering.
In that case, return it to your Pharmacist.
If you are not sure whether you should start having this medicine, talk to your doctor.
Before you start to use it
Tell your doctor or pharmacist if you have allergies to:
- any other medicines
- any other substances, such as foods, preservatives or dyes.
Tell your doctor if you are pregnant or intend to become pregnant. Ask your doctor about the risks and benefits involved.
Tell your doctor or pharmacist if you are breast-feeding or plan to breast-feed.
As morphine passes into breast milk, breast-feeding is not recommended while you are being given morphine.
Tell your doctor if you have or have had any of the following medical conditions:
- epilepsy, convulsions, fits or seizures
- under-active thyroid gland (hypothyroidism) and/or adrenal gland (Addison’s disease)
- enlarged prostate or problems with urination
- tachycardia, fast heart beat
- liver problems
- kidney problems
- any bowel disorders or ulcerative colitis,
- biliary tract disease or inflammation of the pancreas
- myasthenia gravis
- snoring or sleep apnoea (you temporarily stop breathing or have difficulty breathing while asleep
- long-standing pain not related to cancer
- unexplained increase in pain, increased levels of pain with increasing opioid medication or sensitivity not associated with the original pain.
Tell your doctor or pharmacist if you are pregnant or plan to become pregnant or are breast-feeding or plan to breast-feed.
Your doctor or pharmacist can discuss with you the risks and benefits involved.
As morphine passes into breast milk, breast-feeding is not recommended while you are being given morphine.
Your doctor may not want to give you this medicine or may want to take special precautions if you have any of the above conditions.
If you have not told your doctor about any of these things, tell him/her before you take Morphine Sulfate injection.
Addiction
You can become addicted to morphine sulfate even if you use it exactly as prescribed. Morphine sulfate may become habit forming causing mental and physical dependence. If abused it may become less able to reduce pain.
Dependence
As with all other opioid containing products, your body may become used to you taking morphine sulfate. Using it may result in physical dependence. Physical dependence means that you may experience withdrawal symptoms if you stop taking morphine sulfate suddenly, so it is important to take it exactly as directed by your doctor.
Tolerance
Tolerance to morphine sulfate may develop, which means that the effect of the medicine may decrease. If this happens, more may be needed to maintain the same effect.
Withdrawal
Continue using your medicine for as long as your doctor tells you. If you stop having this medicine suddenly, your pain may worsen and you may experience some or all of the following withdrawal symptoms:
- nervousness, restlessness, agitation, trouble sleeping or anxiety
- body aches, weakness or stomach cramps
- loss of appetite, nausea, vomiting or diarrhoea
- increased heart rate, breathing rate or pupil size
- watery eyes, runny nose, chills or yawning
- increased sweating.
Morphine Sulfate injection given to the mother during labour can cause breathing problems and signs of withdrawal in the newborn.
Taking other medicines
Tell your doctor if you are taking any other medicines, including medicines that you buy without a prescription from a pharmacy, supermarket or health food shop.
Some medicines and Morphine Sulfate injection may interfere with each other. These include:
- antidepressants or medicines for anxiety disorders, such as:
- selective serotonin reuptake inhibitors (SSRIs) or
- serotonin and norepinephrine reuptake inhibitors (SNRIs)
- tricyclic antidepressants (TCAs)
- monoamine oxidase inhibitors (MAOIs) i.e. moclobemide, phenelzine, tranylcypromine
- medicines used for migraine (triptans)
- medicines used to prevent or treat nausea and vomiting (5-HT3 receptor antagonists)
- selegeline, a monoamine oxidase inhibitor used to treat Parkinson’s disease
- alcohol
- cimetidine (Magicul), a medicine used to treat stomach or duodenal ulcers, or reflux
- diuretics (fluid tablets)
- other medicines which may make you drowsy such as sleeping tablets, tablets to calm your nerves, sedatives, tranquilisers, hypnotics and muscle relaxants
- anti-diarrhoeal medications
- medicines to treat mental disorders (antipsychotics), other opioid analgesics or strong painkillers
- some antihistamines and some heart medications (e.g. beta blocker)
- benzodiazepines (and other medicines) to treat anxiety, acute stress reactions, agitation, tremor, such as diazepam (Valium), alprazolam or lorazepam
- medicines that lower your blood pressure (antihypertensives)
- warfarin (Marevan, Coumadin), a medicine used to thin the blood
- zidovudine (Retrovir, Combivir, Trizivir) a medicine used to treat HIV infection, ritonavir (Kaletra, Norvir), a medicine used to treat HIV infection
- medications used to reduce risk of blood clots or stroke (e.g. clopidogrel, prasugrel and ticagrelor)
- medications used for seizures such as gabapentinoids (e.g. gabapentin and pregabalin)
- cannibis.
Your doctor will minimise the dose and duration of use; and monitor you for signs and symptoms of breathing difficulties and sedation. You must not drink alcohol while using morphine sulfate.
These medicines may be affected by morphine or may affect how well it works.
You may need to take different amounts of your medicines or you may need to take different medicines.
Your doctor and pharmacist have more information on medicines to be careful with or avoid while you are receiving Morphine Sulfate injection.
Taking Morphine Sulfate injection
How much is given
Your doctor will decide what dose of morphine you will receive. This depends on your condition and other factors, such as your weight.
How is it given
Your doctor or nurse will usually give morphine to you.
Morphine can be given as:
- an injection into a muscle
- a slow injection into a vein
- an injection under the skin or
- by a method called patient-controlled analgesia; this method allows you, the patient, to control the amount of morphine you wish to receive. On experiencing pain, you can press a button, which allows a dose of morphine to be administered to you. To prevent you receiving too much morphine, there is a “lockout” period built into the pump which prevents continuous injection of morphine.
Your doctor will decide the most appropriate way for you to be given morphine.
If you have too much (Overdose)
If you have received too much morphine, you may have symptoms which include severe drowsiness, slow or troubled breathing, severe weakness, slow heart-beat, pale and cold skin.
If you or someone else receive too much (overdose), and experience one or more of the symptoms below, call triple zero (000) for an ambulance. Keep the person awake by talking to them or gently shaking them every now and then. You should follow the above steps even if someone other than you have accidentally used morphine sulfate that was prescribed for you. If someone has an overdose they may experience one or more of the following symptoms:
- Slow, unusual or difficult breathing
- Drowsiness, dizziness or unconsciousness
- Slow or weak heartbeat
- Nausea or vomiting
- Convulsions or fits.
If you think you or someone else may have used too much morphine, you should immediately:
- phone the Poisons Information Centre (by calling 13 11 26), or
- contact your doctor, or
- go to the Emergency Department at your nearest hospital.
You should do this even if there are no signs of discomfort or poisoning.
When seeking medical attention, take this leaflet and remaining medicine with you to show the doctor. Also tell them about any other medicines or alcohol which have been taken.
While you are being given Morphine Sulfate injection
Things you must do
If you are about to be started on any new medicine, tell your doctor, dentist or pharmacist that you are being given morphine. Tell any other doctors, dentists, and pharmacists who treats you that you are being given morphine.
If you plan to have surgery that needs a general anaesthetic, tell your doctor or dentist that you are being given morphine.
If you become pregnant while you are having this medicine, tell your doctor or pharmacist immediately.
Your doctor can discuss with you the risks of being given this medicine while you are pregnant.
Things you must not do
Do not give this medicine to anyone else, even if their symptoms seem to be the same as yours.
Do not use Morphine Sulfate injection to treat any other complaints unless your doctor tells you to.
Do not stop using morphine or lower the dosage without checking with your doctor or pharmacist.
If you have been using morphine for more than two weeks, you may experience unpleasant feelings if you stop morphine suddenly.
Your doctor will probably want you to gradually reduce the amount of morphine you are using, before stopping it completely.
Do not take any other medicines, whether they are prescription or over-the- counter medicines, unless they have been approved or recommended by a doctor or pharmacist who knows you are being given morphine.
Things to be careful of
Be careful driving, operating machinery or doing jobs that require you to be alert while you are having Morphine Sulfate injection until you know how it affects you.
Morphine may cause drowsiness, and impairment of co-ordination, in some people. Make sure you know how you react to morphine. Do not drive a car, operate machinery, or do anything else that could be dangerous if you are drowsy or feeling uncoordinated.
Do not drink alcohol, while you are undergoing treatment with morphine, unless otherwise advised by your doctor or pharmacist, as drowsiness and coordination impairment may be worse.
As morphine may cause nausea and vomiting, your doctor is likely to prescribe medicine for you to take/receive before the morphine, to stop you feeling sick.
Morphine may also cause constipation, so your doctor is likely to prescribe laxatives to prevent this happening.
Tell your doctor, pharmacist or nurse if you have any concerns about being given morphine.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are having Morphine Sulfate injection.
Morphine helps most people with severe pain, but it may have unwanted side effects in a few people.
All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical treatment if you get some of the side effects. If you are over 65 years of age you may have an increased chance of getting side effects.
Do not be alarmed by this list of possible side effects. You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
If you get any side effects, do not stop using morphine without first talking to your doctor or pharmacist.
Tell your doctor or pharmacist if you notice any of the following and they worry you:
- drowsiness, dizziness or unsteadiness
- light-headedness
- confusion
- sweating or flushing
- nausea and/or vomiting
- constipation
- pain and irritation at the injection site
- blurred vision
- dry mouth
- mood changes
- red, itchy skin.
These are the more common side effects of Morphine Sulfate injection. Mostly they are mild and short- lived.
Tell your doctor or pharmacist immediately or go to the Accident and Emergency department at your nearest hospital if you notice any of the following:
- any signs of an allergic reaction to morphine (which are listed at the start of this leaflet)
- severe drowsiness
- slow or troubled breathing
- severe weakness
- agitation
- hallucinations
- seizures (fits)
- unconsciousness
- slow or rapid heart beat
- difficulty in urinating.
The above side effects could be very serious. You may need urgent medical attention or hospitalisation.
Tell your doctor if you notice anything else that is making you feel unwell, even if it is not on this list.
Some people may get other side effects of Morphine Sulfate injection.
After being given Morphine Sulfate injection
Storage
Morphine Sulfate injection will be stored in the pharmacy or on the ward.
Product is for single use in one patient only. Discard any residue.
Keep your ampoules in the cardboard carton until it is time to use them.
Store the ampoules in a cool dry place below 25 degrees celcius (room temperature). Protect from light. Store in original container.
Do not store this medicine or any other medicine in the bathroom or near a sink.
Do not leave it in the car or on window sills.
Heat and dampness can destroy some medicines.
Keep the ampoules where young children cannot reach them.
A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If you no longer need this medicine or it has passed its expiry date, return any unused ampoules to your pharmacist.
Product description
What it looks like
Morphine Sulfate injection comes in amber glass 1mL ampoule containing a clear colourless to slightly yellow solution.
There are different strengths available:
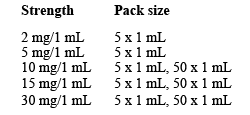
Not all presentations and/or packs are marketed.
Ingredients
As well as morphine sulfate pentahydrate, the active ingredient, the ampoules also contain sodium chloride, hydrochloric acid (to adjust pH) and water for injection.
Sponsor
Australian Sponsor
Medicianz Healthcare Pty Limited
Unit 2, 6-7 Gilda Court
MULGRAVE
VICTORIA 3170
Marketed and distributed by 
Medsurge Healthcare Pty Ltd
Tel: 1300 788 261
www.medsurge.com.au
Registration Numbers
AUST R 308716 (2 mg/1 mL)
AUST R 308714 (5 mg/1 mL)
AUST R 308718 (10 mg/1 mL)
AUST R 308715 (15 mg/1 mL)
AUST R 308721 (30 mg/1 mL)
This leaflet was prepared in December 2020.
