What is in this leaflet
This leaflet answers some common questions about OMNARIS.
It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
The information in this leaflet was last updated on the date listed on the final page. More recent information on the medicine may be available. You should ensure that you speak to your pharmacist or doctor to obtain the most up to date information on this medicine. Those updates may contain important information about the medicine and its use of which you should be aware.
All medicines have risks and benefits. Your doctor has weighed the risks of you using this medicine against the benefits they expect it will have for you.
If you have any concerns about using this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine. You may need to read it again.
What OMNARIS is used for
The medicine is sprayed into the nose to help treat symptoms of seasonal allergic rhinitis in adults and children 6 years of age and older, and perennial allergic rhinitis in adults and adolescents 12 years of age and older.
Allergic rhinitis (hay fever) is an inflammation or swelling of the nose lining, which may cause blockage, runny nose, itching and/or sneezing.
You may have symptoms only during spring or summer (seasonal). This type of allergy is generally due to various pollens. Some people may experience symptoms all year round (perennial). This is usually caused by house dust mites, pets or moulds.
The medicine contains ciclesonide. This belongs to a family of medicines called corticosteroids, which are used to help reduce inflammation.
Ask your doctor if you have any questions about why it has been prescribed for you. Your doctor may have prescribed it for another purpose.
This medicine is not addictive.
This medicine is available only with a doctor's prescription.
Before you use OMNARIS
When you must not use it
Do not use OMNARIS if you have an allergy to:
- any medicine containing ciclesonide or any other corticosteroid medicines
- any of the ingredients listed at the end of this leaflet
Some symptoms of an allergic reaction include skin rash, itching, shortness of breath or swelling of the face, lips or tongue, which may cause difficulty in swallowing or breathing.
Do not give OMNARIS to a child under the age of 6 years.
Do not use it after the expiry date printed on the pack or if the packaging is damaged or shows signs of tampering. If it has expired or is damaged return it to your pharmacist for disposal.
If you are not sure whether you should start using this medicine, talk to your doctor or pharmacist.
Before you start to use it
Tell your doctor if you have allergies to any other medicines, foods, preservatives or dyes.
Tell your doctor if you have or have had any of the following medical conditions:
- frequent nose bleeds
- nasal or sinus infection
- recent injury or surgery to your nose
- ulcers or sores in your nose
- any type of infection caused by bacteria, virus, fungus, or parasites
- a history of tuberculosis (TB) infections
- herpes simplex (virus) infection of the eye.
Tell your doctor if you are pregnant, intend to become pregnant, or are breastfeeding Your doctor or pharmacist can discuss the risks and benefits involved.
If you have not told your doctor about any of the above, tell him/her before you start using OMNARIS.
Taking other medicines
Tell your doctor or pharmacist if you are taking any other medicines, including any that you buy without a prescription from your pharmacy, supermarket or health food store.
Some medicines and OMNARIS may interfere with each other. These include:
- other corticosteroid medicines such as tablets, asthma inhalers, nasal sprays, or eye/nose drops
- medicines that suppress the immune system (e.g. cyclosporine)
- some antifungal medicines (e.g. ketoconazole, itraconazole)
- some antiviral medicines (e.g. ritonavir, nelfinavir).
These medicines may be affected by OMNARIS or may affect how well it works. You may need to use different amounts of your medicine or use different medicines.
Your doctor or pharmacist has more information on medicines to be careful with or to avoid while taking OMNARIS.
How to use OMNARIS
- Remove OMNARIS from its foil pouch. Count 4 months from this date and write the date (that is 4 months from removing the bottle from the foil pouch) on the space provided on the nasal spray bottle. It is important to throw away the nasal spray bottle after this date.
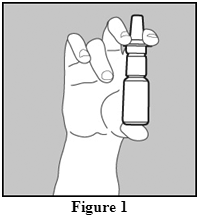
- Before you use OMNARIS for the first time, you will need to prime the bottle. Hold the bottle upright and shake the bottle gently. To prime OMNARIS Nasal Spray, fully press down on the finger rests of the applicator eight times (See Figure 1). If you do not use the nasal spray for 4 days, you will need to shake the bottle gently, and prime the pump again by spraying one time, or until you see a fine mist.
Using the spray
- Gently blow your nose to clear your nostrils if needed
- Shake the bottle gently and remove the cap
- Hold the bottle firmly with your index and middle finger on either side of the nozzle while supporting the base of the bottle with your thumb (Figure 1)
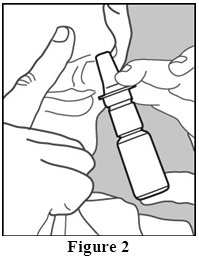
- Insert the nozzle tip into one nostril, and close the other nostril with your finger (Figure 2)
- Tilt your head forward slightly and keeping the bottle upright, press the pump quickly and firmly and inhale through your nose as you spray (Figure 3).
Do not spray in eyes or directly onto the nasal septum (the wall between the two nostrils).
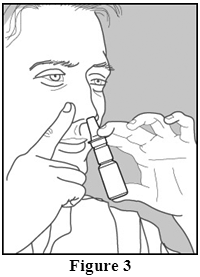
- Repeat steps 3-5 for the second spray in the same nostril and for each spray in the other nostril.
How to clean it
After daily use of your nasal spray, wipe the nozzle tip with a clean tissue and replace the cap.
If the nozzle is clogged or requires more thorough cleaning, use the following cleaning instructions:
- Remove the cap, grasp the white plastic pump firmly with one hand and then carefully pull upwards on the shoulders of the nozzle with the other hand to free the nozzle
- Wash the cap and nozzle with warm water
- Dry and replace the nozzle. The nozzle will snap into place when properly positioned
- Prime the pump by spraying one time or until a fine mist appears
- Replace the cap.
Do not try to unblock the tiny spray hole on the nozzle tip with a pin or other sharp object.
Do not twist or attempt to remove the white plastic pump attached to the medication bottle.
How to know when your nasal spray bottle is empty
The amount of nasal spray left can be seen through a window on the bottle. You should discard the bottle after using 120 sprays (or 60 sprays if your bottle is a 60 spray pack) or 4 months after opening the foil pouch, whichever comes first.
Follow all directions given to you by your doctor or pharmacist carefully. They may differ from the information contained in this leaflet.
If you do not understand the instructions, ask your doctor or pharmacist for help.
How much to use
The recommended dosage is 2 sprays in each nostril once daily.
The maximum total daily dosage should not exceed 2 sprays in each nostril (200 micrograms per day).
Do not use more than the recommended dose.
Your doctor may change this dosage, depending on your response to this medicine.
Use the lowest dose that controls your symptoms.
How long to use it
Continue using your medicine for as long as your doctor tells you.
If you forget to take it
If you miss a dose, use OMNARIS when the next dose is due.
Do not take a double dose to make up for the dose that you missed. This may increase the chance of getting an unwanted side effect.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering when to take your medicine, ask your pharmacist for hints.
If you take too much (overdose)
If you or someone else accidentally uses too much OMNARIS on one occasion, there is nothing to worry about.
However, if you use too much of it over a long time (months or years), you may start to get unwanted side effects inside your nose and body.
Discuss any worries you may have about this with your doctor or pharmacist.
Immediately telephone your doctor, or the Poisons Information Centre (telephone 13 11 26) if you think you or anyone else may have used too much OMNARIS. Do this even if there are no signs of discomfort or poisoning.
While you are using OMNARIS
Things you must do
Keep all of your doctor's appointments so that your progress can be checked.
Ask your doctor to examine your nose from time to time to make sure the medicine is working and to prevent unwanted side effects.
If you are about to be started on any new medicine, remind your doctor and pharmacist that you are using this medicine.
Tell any other doctors, dentists and pharmacists who treat you that you are using this medicine.
If you are going to have surgery, tell the surgeon or anaesthetist that you are using this medicine. It may affect other medicines used during surgery.
If you become pregnant while you are taking this medicine, tell your doctor immediately.
Things you must not do
Do not use this medicine to treat any other complaints unless your doctor or pharmacist tells you to.
Do not give this medicine to anyone else, even if they have the same condition as you.
Things that may be helpful
If possible, avoid situations that you know will trigger your symptoms.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are using OMNARIS.
It helps most people with seasonal and perennial allergic rhinitis, but it may have unwanted side effects in a few people. All medicines have some unwanted side effects. Sometimes they are serious, but most of the time they are not. You may need medical attention if you get some of the side-effects.
Do not be alarmed by this list of possible side effects. You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor or pharmacist if you notice any of the following and they worry you:
- headache
- nose bleeds
- burning or irritation inside the nose
- irritated throat.
The above list includes the more common side effects of your medicine. They are usually mild and short-lived.
Tell your doctor as soon as possible if you notice any of the following:
- frequent nose bleeds
- signs or symptoms of a nasal or sinus infection such as fever, pain or swelling, or discoloured nasal discharge
- allergic reactions such as swelling of the face, lips, tongue or other parts of the body, loss of consciousness, shortness of breath, or nasal congestion.
The above list includes serious side effects that may require medical attention. Serious side effects are rare.
Tell your doctor if you notice any issues with your eyes such as blurred vision or other problems with your eyesight. Your doctor may need to send you to an ophthalmologist (eye doctor) to check that you don't have eye problems such as cataracts (clouding of the eye lens), glaucoma (increased pressure in your eyeballs) or other rare eye conditions reported with corticosteroid use.
In rare cases, corticosteroids taken through the nose for long periods can affect how children grow. If your child uses OMNARIS for a long period of time, your doctor may need to monitor your child's height.
Tell your doctor or pharmacist if you notice anything else that is making you feel unwell. Other side effects not listed above may occur in some people.
After using OMNARIS
Storage
Keep OMNARIS in a cool, dry place where the temperature stays below 30°C. Do not freeze the bottle.
Do not store it or any other medicine in the bathroom, near a sink, or on a windowsill. Do not leave it in the car. Heat and dampness can destroy some medicines.
Keep it where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor or pharmacist tells you to stop taking this medicine, or the medicine has passed its expiry date, ask your pharmacist what to do with any that are left over.
Discard the bottle after using 60 or 120 sprays, or 4 months after opening the foil pouch, whichever comes first.
Product description
What it looks like
OMNARIS nasal spray is a white to off-white suspension contained in a brown glass bottle, fitted with a manual spray pump. The bottle is enclosed in a foil pouch.
Also enclosed within the foil pouch is an oxygen-absorber sachet, this is included to help protect the product prior to use and should be discarded with the foil pouch after opening.
OMNARIS is registered* in pack sizes of 60 or 120 sprays.
*not all registered pack sizes might be available in Australia
Ingredients
OMNARIS contains ciclesonide as the active ingredient. Each spray delivers 50 micrograms of ciclesonide.
OMNARIS also contains the following inactive ingredients:
- microcrystalline cellulose
- carmellose sodium
- hypromellose
- potassium sorbate
- disodium edetate
- purified water
- hydrochloric acid to adjust pH of the solution.
- nitrogen
OMNARIS does not contain gluten, sucrose, lactose, tartrazine or any other azo dyes.
Supplier
Chiesi Australia Pty Ltd
Suite 3, 22 Gillman Street,
Hawthorn East, VIC 3123
Email: medicalaffairs.au@chiesi.com
Website: www.chiesi.com.au
This leaflet was prepared on September 2021
Australian Registration Number
AUST R 167910
Published by MIMS November 2021
