Boxed Warnings
Limitations of use. Because of the risks associated with the use of opioids, Panamax Co should only be used in patients for whom other treatment options, including non-opioid analgesics, are ineffective, not tolerated or otherwise inadequate to provide appropriate management of pain (see Section 4.4 Special Warnings and Precautions for Use).
Hazardous and harmful use. Panamax Co poses risks of hazardous and harmful use which can lead to overdose and death. Assess the patient's risk of hazardous and harmful use before prescribing and monitor the patient regularly during treatment (see Section 4.4 Special Warnings and Precautions for Use).
Life threatening respiratory depression. Serious, life-threatening or fatal respiratory depression may occur with the use of Panamax Co. Be aware of situations which increase the risk of respiratory depression, modify dosing in patients at risk and monitor patients closely, especially on initiation or following a dose increase (see Section 4.4 Special Warnings and Precautions for Use).
Concomitant use of benzodiazepines and other central nervous system (CNS) depressants, including alcohol. Concomitant use of opioids with benzodiazepines, gabapentinoids, antihistamines, tricyclic antidepressants, antipsychotics, cannabis or other central nervous system (CNS) depressants, including alcohol, may result in profound sedation, respiratory depression, coma, and death. Limit dosages and durations to the minimum required; and monitor patients for signs and symptoms of respiratory depression and sedation. Caution patients not to drink alcohol while taking Panamax Co.
1 Name of Medicine
Paracetamol and codeine phosphate hemihydrate.
2 Qualitative and Quantitative Composition
Each tablet contains: paracetamol 500 mg, codeine phosphate hemihydrate 8 mg.
Excipients with known effect. Potassium sorbate.
For the full list of excipients, see Section 6.1 List of Excipients.
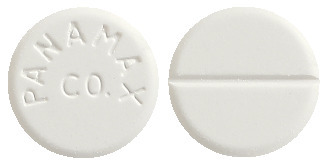
3 Pharmaceutical Form
Tablets (white, scored, marked PANAMAX CO).
4 Clinical Particulars
4.9 Overdose
Elderly persons, small children, patients with liver disorders, chronic alcohol consumption or chronic malnutrition, as well as patients concomitantly treated with enzyme-inducing drugs are at an increased risk of intoxication, including fatal outcome.
Symptoms. Toxic symptoms include vomiting, abdominal pain, hypotension, sweating, central stimulation with exhilaration and convulsions in children, drowsiness, respiratory depression, cyanosis and coma. Nausea, vomiting, anorexia, pallor and abdominal pain generally appear during the first 24 hours of overdosage with paracetamol. Overdosage with paracetamol may cause hepatic cytolysis which can lead to hepatocellular insufficiency, gastrointestinal bleeding, metabolic acidosis, encephalopathy, disseminated intravascular coagulation, coma and death. Increased levels of hepatic transaminases, lactate dehydrogenase and bilirubin with a reduction in prothrombin level can appear 12 to 48 hours after acute overdosage. It can also lead to pancreatitis, acute renal failure and pancytopenia. The most serious adverse effect of acute overdosage of paracetamol is a dose dependent, potentially fatal hepatic necrosis. In adults, hepatotoxicity may occur after ingestion of a single dose of 10 to 15 g (30 tablets) of paracetamol; a dose of 25 g (50 tablets) or more is potentially fatal. Symptoms during the first two days of acute poisoning by paracetamol do not reflect the potential seriousness of the intoxication. Major manifestations of liver failure such as jaundice, hypoglycaemia and metabolic acidosis may take at least three days to develop.
The ingestion of very high doses of codeine can cause initial excitation, anxiety, insomnia followed by drowsiness in certain cases, areflexia progressing to stupor or coma, headache, miosis, alterations in blood pressure, arrhythmias, dry mouth, hypersensitivity reactions, cold clammy skin, bradycardia, tachycardia, convulsions, gastrointestinal disorders, nausea, vomiting and respiratory depression.
Severe intoxication can lead to apnoea, circulatory collapse, cardiac arrest and death.
Treatment. Despite lack of significant early symptoms, patients should be referred to hospital urgently for immediate medical attention.
Consists primarily of management of paracetamol toxicity; naloxone is the treatment of choice for codeine intoxication.
Determinations of the plasma concentration of paracetamol are recommended.
Plasma concentration of paracetamol should be measured at 4 hours or later after ingestion (earlier concentrations are unreliable).
Where paracetamol intoxication is suspected, intravenous administration of SH group donators such as acetylcysteine within the first 10 hours after ingestion is indicated. Although acetylcysteine is most effective if initiated within this period, it can still offer some degree of protection if given as late as 48 hours after ingestion; in this case it is taken for longer.
In cases of overdosage, methods of reducing the absorption of ingested drug are important.
Prompt administration of 50 g activated charcoal and 500 mL iced mannitol 20% by mouth may reduce absorption.
If the history suggests that 15 g paracetamol or more has been ingested, administer one of the following antidotes:
Acetylcysteine 20% i.v. Administer 20% acetylcysteine (Parvolex, David Bull) immediately without waiting for positive urine test or plasma level results: initial dose 150 mg/kg over 15 minutes, followed by continuous infusion of 50 mg/kg in 500 mL 5% glucose over 4 hours and 100 mg/kg in 1 L 5% glucose over 16 hours or;
Oral methionine. 2.5 g immediately followed by three further doses of 2.5 g at four hourly intervals. For a 3-year-old child, 1 g methionine 4-hourly for four doses has been used.
If more than ten hours have elapsed since the overdosage was taken, the antidote may be ineffective.
Relating to codeine component. In general, treatment should be symptomatic: re-establish adequate respiratory exchange by ensuring a clear airway and using mechanical ventilation. When treatment for paracetamol toxicity has been initiated the opioid antagonist naloxone hydrochloride is an antidote to respiratory depression; naloxone 400 microgram may be administered SC, IM or IV; IV may be repeated at intervals of 2 to 3 minutes if necessary. Assisted respiration may be required.
Further measures will depend on the severity, nature and course of clinical symptoms of intoxication and should follow standard intensive care protocols.
For information on the management of overdose contact the Poisons Information Centre on 131126 (Australia).
5 Pharmacological Properties
5.3 Preclinical Safety Data
Genotoxicity. No data available.
Carcinogenicity. No data available.
6 Pharmaceutical Particulars
6.7 Physicochemical Properties
Chemical structure.
https://stagingapi.mims.com/au/public/v2/images/fullchemgif/CSPARCET.gif https://stagingapi.mims.com/au/public/v2/images/fullchemgif/CSCODPHH.gif Paracetamol MW: 151.17. Codeine phosphate hemihydrate MW: 406.37.
CAS number. CAS: 103-90-2 (paracetamol). CAS: 41444-62-6 (codeine phosphate hemihydrate).
7 Medicine Schedule (Poisons Standard)
Prescription Only Medicine (40s) (Schedule 4).
Summary Table of Changes
https://stagingapi.mims.com/au/public/v2/images/fulltablegif/PANACOST.gif