What is in this leaflet
This leaflet answers some common questions about PHYSEPTONE. It does not contain all of the available information.
It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking PHYSEPTONE against the benefits this medicine is expected to have for you.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine.
You may need to read it again.
What PHYSEPTONE is used for
PHYSEPTONE contains methadone hydrochloride, which belongs to a group of medicines called opioid analgesics.
PHYSEPTONE is used for the relief of chronic, moderate to severe pain.
Addiction
You can become addicted to PHYSEPTONE tablets even if you take it exactly as prescribed. PHYSEPTONE tablets may become habit forming causing mental and physical dependence. If abused, it may become less able to reduce pain.
Dependence
As with all other opioid containing products, your body may become used to you taking this medicine. Taking it may result in physical dependence. Physical dependence means that you may experience withdrawal symptoms if you stop taking PHYSEPTONE tablets suddenly, so it is important to take it exactly as directed by your doctor.
Tolerance
Tolerance to PHYSEPTONE tablets may develop, which means that the effect of the medicine may decrease. If this happens, more may be needed to maintain the same effect.
Withdrawal
Continue taking your medicine for as long as your doctor tells you.
If you stop having this medicine suddenly, your pain may worsen and you may experience some or all of the following withdrawal symptoms:
- nervousness, restlessness, agitation, trouble sleeping or anxiety
- body aches, weakness or stomach cramps
- loss of appetite, nausea, vomiting or diarrhoea
- increased heart rate, breathing rate or pupil size
- watery eyes, runny nose, chills or yawning
- increased sweating.
PHYSEPTONE tablets given to the mother during labour can cause breathing problems and signs of withdrawal in the newborn.
Ask your doctor if you have any questions about why PHYSEPTONE has been prescribed for you.
Your doctor may have prescribed it for another reason.
PHYSEPTONE tablets is only available with a doctor’s prescription.
Before you take it
When you must not take it:
Do not take PHYSEPTONE if:
- you have ever had an allergic reaction to methadone hydrochloride (the active ingredient in PHYSEPTONE tablets); any other opioid drug; or any of the ingredients listed at the end of this leaflet.
- you have any other medical condition including:
- suffering from a lung disorder such as asthma, or any illness causing difficulty in breathing, especially if there is excessive phlegm or skin is bluish in colour
- a recent head injury, or increased pressure in the head
- a bowel condition known as ulcerative colitis
- certain liver or kidney conditions
- certain heart conditions
- alcoholism.
- you are taking or have recently taken antidepressants of the type called monoamine oxidase inhibitors (MAOIs)
- you suffer from biliary and renal tract spasm
- the expiry date (EXP) printed on the pack has passed
- the packaging is torn or shows signs of tampering.
Before you start to take it
Tell your doctor if:
- you are allergic to foods, dyes, preservatives or any other medicines
- you are pregnant, or become pregnant while taking PHYSEPTONE, are about to give birth, or are breastfeeding
- you have any other medical condition including:
- hormone problems
- diabetes
- prostate disease
- phaeochromocytoma (a rare tumour of the adrenal gland).
Symptoms include bouts of anxiety and headaches. There may be palpitations (banging of the heart felt in the chest), dizziness, weakness, nausea, vomiting, diarrhoea, dilated pupils, blurry vision, stomach pains and raised blood pressure.
- you are taking any other medicines or intend to drink alcohol while you are taking PHYSEPTONE.
These include medicines that you buy without a prescription from a pharmacy, supermarket or health food shop, including St John’s Wort.
Your doctor or pharmacist will have a complete list of medicines that may cause problems when taken with PHYSEPTONE tablets.
How to take it
How much to take
Follow all directions given to you by your doctor and pharmacist carefully.
The usual dose for adults is half to one tablet taken 2 to 3 times a day, but this dosage may be adjusted by your doctor.
Do not take more than the recommended dose.
It can result in low blood sugar.
How to take it
Swallow the tablets with a glass of water.
How long to take it for
Do not stop taking PHYSEPTONE or change the dose without first checking with your doctor.
Use in children and elderly patients
PHYSEPTONE tablets are not recommended for use in children. The doctor may prescribe a smaller dose in elderly patients.
If you take too much (overdose)
If you or someone else receive too much (overdose) and experience one or more of the symptoms below, immediately call triple zero (000) for an ambulance. Keep the person awake by talking to them or gently shaking them every now and then. You should follow the above steps even if someone other than you have accidentally taken PHYSEPTONE tablets that was prescribed for you. If someone takes an overdose they may experience one or more of the following symptoms:
- slow, unusual or difficult breathing
- drowsiness, dizziness or unconsciousness
- slow or weak heartbeat
- nausea or vomiting
- convulsions or fits.
If you think you or someone else may have used too much PHYSEPTONE tablets, you should immediately:
- phone the Poisons Information Centre (by calling 13 11 26), or
- contact your doctor, or
- go to the Emergency Department at your nearest hospital.
You should do this even if there are no signs of discomfort or poisoning.
When seeking medical attention, take this information and remaining medicine with you to show the doctor. Also tell them about any other medicines or alcohol which have been taken.
While you are taking it
Things you must do
Tell your doctor if, for any reason, you have not taken your medicine exactly as directed.
Otherwise, your doctor may think that it was not working as it should and change your treatment unnecessarily.
If you forget to take it
If you forget to take a dose, take it as soon as you remember and then go on as before, but remember not to take the tablets more often than recommended by your doctor.
Things you must not do
Do not give this medicine to anyone else, even if their symptoms seem similar to yours.
Seek medical help immediately if PHYSEPTONE is accidentally taken by a child.
Do not use PHYSEPTONE to treat any other complaints unless your doctor says to.
Things to be careful of
PHYSEPTONE tablets may cause drowsiness. It is recommended that you don't drive, use machinery or undertake any activities where alertness is required.
It is unwise to drink alcohol while taking PHYSEPTONE.
Particular care should be taken when starting treatment with PHYSEPTONE or increasing the dose.
PHYSEPTONE can decrease heart and breathing rates, which if severe may lead to death.
Speak to your doctor immediately if you have any concerns.
Side effects
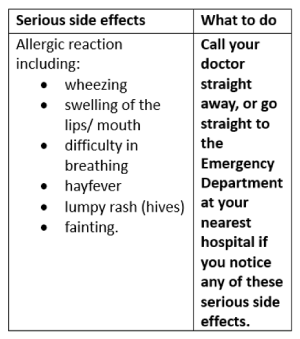
Check with your doctor as soon as possible if you think you are experiencing any side effects or allergic reactions due to taking PHYSEPTONE tablets, even if the problem is not listed below.
Like other medicines, PHYSEPTONE tablets may have unwanted side effects. Sometimes they are serious, most of the time they are not. You may need medical treatment if you get some of the side effects.
With prolonged use, the dose may have to be increased to achieve the same benefit, whilst a sudden decrease in dose or interruption of therapy may give rise to withdrawal symptoms.
Tell your doctor if you notice any of the following and they worry you: 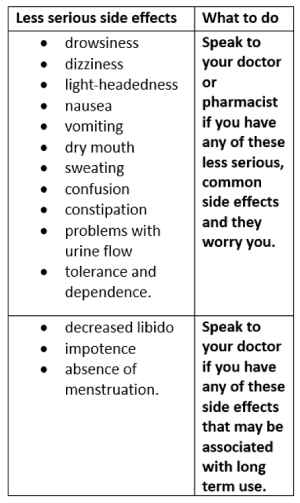
Tell your doctor or pharmacist if you notice anything else that is making you feel unwell.
Other side effects not listed above may occur in some people.
Do not be alarmed by this list of possible side effects.
You may not experience any of them.
After taking it
Storage
Keep PHYSEPTONE tablets in a cool, dry place, protected from light, where the temperature stays below 25°C.
Keep medicines where children cannot reach them.
A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Do not store it or any other medicine in the bathroom or near a sink. Do not leave it in a car, on a window sill or in the bathroom.
Heat and dampness can destroy some medicines.
Keep it in the blister pack until it is time to take them.
Disposal
Return any unused or expired medicine to your pharmacist.
Product description
What it look like
PHYSEPTONE are white, uncoated, round, biconvex tablets, scored on one side. Available in blister packs of 20 tablets.
Ingredients
Active ingredient:
Each PHYSEPTONE tablet contains methadone hydrochloride 10 mg.
Inactive ingredients:
- gelatin
- glycerol
- lactose monohydrate
- maize starch
- magnesium stearate.
Sponsor
Aspen Pharmacare Australia Pty Ltd
34-36 Chandos Street,
St. Leonards NSW 2065
Australia
Australian registration number: AUST R 76083.
This leaflet was revised in December 2024.
