What is in this leaflet
This leaflet answers some common questions about RYALTRIS® nasal spray.
This leaflet does not contain all the available information and it does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you using this medicine against the benefits they expect it will have for you.
If you have any concerns about using this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine. You may want to read it again.
What RYALTRIS® is used for
RYALTRIS® contains the active ingredients olopatadine hydrochloride and mometasone furoate monohydrate. Olopatadine hydrochloride is an antihistamine that reduces the effects of histamine, a natural chemical in the body. Histamine can make you sneeze, have itchy or watery eyes, and runny nose. Mometasone furoate monohydrate belongs to a family of medicines called corticosteroids, which are used to help reduce inflammation.
RYALTRIS® is sprayed into the nose to treat the symptoms of allergic rhinitis (hay fever or other year round allergies) and rhinoconjunctivitis (allergy defined by symptoms in the nose and eyes).
RYALTRIS® relieves symptoms of allergies, including stuffiness (congestion) in the nose, runny nose, itchy nose, sneezing, as well as watering, itching and redness of the eye.
Ask your doctor if you have any questions about why this medicine has been prescribed for you. Your doctor may have prescribed it for another reason.
There is no evidence that this medicine is addictive.
This medicine is available only with a doctor's prescription.
There is not enough information to recommend the use of this medicine for children under the age of 6 years.
Before you use RYALTRIS®
When you must not use it
Do not use the medicine if:
- You have an allergy to:
- any medicine containing olopatadine hydrochloride or mometasone furoate monohydrate
- any of the ingredients listed at the end of this leaflet.
- You have a tendency to bleed or recurrent nose bleeding.
- You have severe nose infection, especially fungal infection.
- You have had recent nose injury or nose surgery; check with your doctor, because you may be told to wait until healing has occurred before using this medicine.
Do not give this medicine to a child under the age of 6 years. Safety and effectiveness in children younger than 6 years have not been established. Safety in children 6 to 11 years of age has not been studied beyond 2 weeks.
Do not use it after the expiry date (EXP) printed on the pack or if the packaging is torn or shows sign of tampering. If it has expired or is damaged, return it to your pharmacist for disposal.
If you are not sure whether you should start taking this medicine, contact your doctor.
Before you start to use it
Tell your doctor or pharmacist if you have or have had any medical conditions, especially the following:
- history of tuberculosis, or active tuberculosis
- tendency to bleed or recurrent nose bleeding
- severe nose infection, especially fungal infection.
- infection of the nose, sinus, mouth, throat, lungs or eye infection caused by herpes
- sores in the nose
- recent injury or surgery to your nose
- non-allergic conditions affecting the inside of your nose, such as a deviated septum
- eye or vision problems, such as cataracts or glaucoma (increased pressure in your eyes)
- been near someone who has chickenpox or measles
- not feeling well or have other symptoms that you do not understand
- have any other medical conditions
Tell your doctor or pharmacist if you are taking other corticosteroid medicines, either by mouth, as eye drops, as an asthma inhaler or by injection.
Tell your doctor or pharmacist if you have allergies to any other medicines, foods, preservatives of dyes.
Tell your doctor if you are pregnant or intend to become pregnant Your doctor will discuss the possible risks and benefits of using this medicine during pregnancy.
Tell your doctor if you are breastfeeding or plan to breastfeed. Breastfeeding is not recommended during treatment with this medicine.
If you have not told your doctor about any of the above, tell them before you use this medicine.
Taking other medicines
Tell your doctor if you are taking any other medicines, including any that you buy without a prescription from your pharmacy, supermarket or health food shop.
Some medicines and mometasone furoate monohydrate may interfere with each other. These include:
- Certain antifungals such as ketoconazole and itraconazole
- Some antibiotics such as clarithromycin
- Some medicines used to treat HIV infection such as ritonavir and cobcistat-containing products.
How to use RYALTRIS®
For use in your nose only. Do not spray in your eyes or mouth.
Read the Instructions for Use before you start to use RYALTRIS® and each time you get a refill. There may be new information. This leaflet does not take the place of talking with your healthcare provider about your medical condition or treatment. Before you use RYALTRIS®, make sure your healthcare provider shows you the right way to use it. Shake the bottle well before each use.
Follow all directions given to you by your doctor or pharmacist carefully and do not use more than the recommended dose. These directions may differ from the information contained in this leaflet.
If you do not understand the instructions, ask your doctor or pharmacist for help.
How much to use
Hay fever and allergies in adults (including the elderly) and children 12 years of age and older:
Two sprays into each nostril twice daily (morning and evening).
Hay fever and allergies in children 6 to 11 years of age:
One spray into each nostril twice daily (morning and evening).
If symptoms worsen during treatment, see your doctor immediately.
About your nasal spray
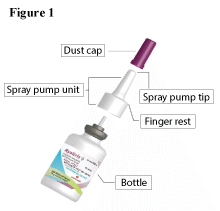
Your RYALTRIS® nasal spray bottle (see Figure 1)
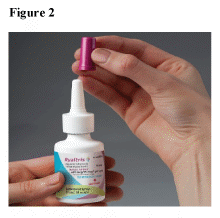
Priming your RYALTRIS® pump before first use (Step 1 and 2)
Before you use RYALTRIS® for the first time, you will need to shake the bottle well and prime the pump.
Before you prime the bottle, shake the bottle well.
Step 1. Remove the dust cap.
Shake the bottle well. Remove the purple plastic dust cap from the spray pump tip of the bottle. (See Figure 2)
Step 2. Preparing the nasal spray bottle
- Hold the nasal spray bottle firmly and upright with your index and middle finger on either side of the applicator (on finger rests) while supporting the grooved base of the bottle with your thumb.
- Before you use the bottle for the first time, push down on the pump quickly and firmly 6 times, releasing the spray into the air, away from the eyes and face until a fine mist appears. (See Figure 3)

- If you do not use RYALTRIS® for 14 or more days, you will need to shake the bottle well, and prime the pump with 2 sprays or until a fine mist appears.
Your RYALTRIS® is now ready for use.
Using your RYALTRIS®:
Step 3.
Gently blow your nose to clear your nostrils. (See Figure 4)

Step 4. Using the nasal spray:
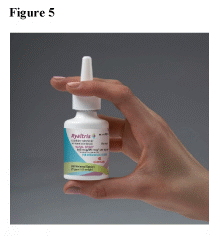
- Shake the bottle well before each use (morning and evening).
- Hold the bottle firmly with your index and middle finger on either side of the applicator (on finger rests) while supporting the grooved base of the bottle with your thumb. (See Figure 5)
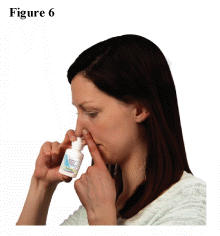
- Hold 1 nostril closed with a finger. Insert the end of the nasal tip into the other nostril, pointing it slightly toward the outside of the nose, away from the nasal septum (the wall between the 2 nostrils). (See Figure 6)
- Tilt your head forward slightly. Keep the bottle upright, and press down once quickly and firmly on the finger rests to activate the pump. Breathe in (inhale) gently through your nose as you spray. Then breathe out through your mouth. (See Figure 7)

- Try not to get any spray in your eyes or directly on your nasal septum (the wall between the 2 nostrils).
- For adults and adolescents (12 years of age and older), repeat the above steps and deliver a second spray in the same nostril. Repeat with 2 sprays in the other nostril.
- Avoid blowing your nose for the next 15 minutes to make sure RYALTRIS® gets a chance to work. Do not tip your head back right after using, this will help keep the medicine from going into your throat.
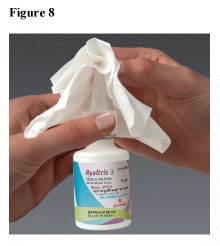
- After you finish using the medicine, wipe the tip with a clean dry tissue or cloth. (See Figure 8)
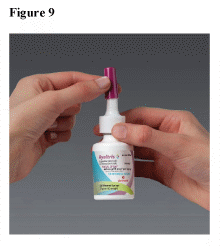
While holding the spray pump unit, push the dust cap back on the spray tip of the bottle until a noticeable click is observed to help prevent nozzle blockage. (See Figure 9)
There are three different bottle sizes of RYALTRIS® containing 240 sprays, 120 sprays or 56 sprays after initial priming.
Keep track of the number of days you use RYALTRIS®. If you are using the 240-spray bottle, throw away the bottle after 240 sprays (30 days of use for adults and adolescents 12 years and older / 60 days of use for children 6 to 11 years old) or upon expiration, whichever comes first.
Similarly, throw away the 120-spray bottle after 120 sprays (15 days of use for adults and adolescents 12 years and older / 30 days of use for children 6 to 11 years old).
If you are using the 56-spray bottle, throw it away after 56 sprays (7 days of use for adults and adolescents 12 years and older / 14 days of use for children 6 to 11 years old).
Even if the bottle seems to have medicine left in it, you may not receive the correct dose.
Do not count any sprays used for initial priming of the bottle.
How to clear the RYALTRIS® spray pump unit if it becomes clogged:
Do not try to unblock the spray pump unit by inserting a pin or other sharp object. This will damage the spray pump unit, and you may not get the correct dose of medicine.
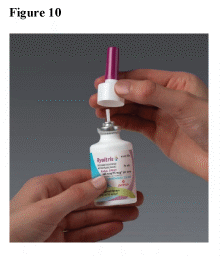
If the spray pump unit becomes blocked, remove it by gently pulling upward. Remove the dust cap and place only the spray pump unit in warm water to soak. (See Figure 10)
After soaking for approximately 15 minutes, rinse the spray pump unit and cap with warm tap water, and allow them to dry completely. (See Figure 11)

When dry, place the dust cap on the spray pump tip and put the spray pump unit back on the bottle. (See Figure 12)
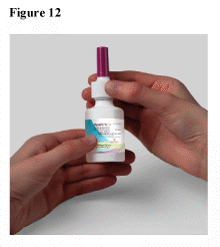
Following the unblocking procedure, review the “Priming your RYALTRIS® pump before use” section above and re-prime using 2 sprays. Replace the dust cap, and your RYALTRIS® is ready for use.
Repeat the unblocking steps if needed.
When to use it
Your doctor will tell you when to use the nasal spray. Do not use more often than instructed.
How long to use it
Your doctor will tell you how long to use it.
If you forget to use it
If it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to.
Otherwise, take it as soon as you remember, and then go back to use your medicines as you would normally.
Do not take a double dose to make up for the dose you missed.
If you are not sure what to do, ask your doctor or pharmacist.
If you take too much (overdose)
Immediately telephone your doctor or the Poisons Information Centre (telephone 13 11 26) for advice, or go to the Accident and Emergency Department at your nearest hospital, if you think you or anyone else may have used too much of this medicine, or if you feel unwell. Do this even if there are no signs of discomfort or poisoning.
Keep telephone numbers for these places handy.
While you are using RYALTRIS®
Things you must do
If you become pregnant while you are using this medicine, tell your doctor.
Tell any other doctors, dentists and pharmacists who are treating you that you are using this medicine.
If you are about to be started on any new medicine, tell your doctor, dentist or pharmacist that you are using this medicine.
If you are going to have surgery, tell the surgeon or anaesthetist that you are using this medicine.
See your doctor immediately if you notice the symptoms of severe bacterial infection. These symptoms may include fever, persistent face/tooth pain on one side of the face, swelling around the eye area, or worsening of symptoms after an initial improvement.
Things you must not do
Do not use this medicine to treat any other complaints unless your doctor tells you to.
Do not give this medicine to anyone else, even if they have the same condition as you.
Do not stop taking your medicine or lower the dosage without checking with your doctor.
Things to be careful of
RYALTRIS® can cause sleepiness or drowsiness. Be careful drinking alcohol or taking any other medicines that may cause you to feel sleepy while using RYALTRIS®.
Do not drive, operate machinery, or do anything that needs you to be alert until you know how RYALTRIS® affects you.
If you have a lower resistance to infection, avoid coming into contact with anyone who has measles or chickenpox, especially while you are using cortisone-type medicines. Tell your doctor if you do.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are using this medicine.
The doctor will decide whether any change in your treatment is needed.
This medicine helps most people, but it may have unwanted side effects in a few people.
All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical treatment if you get some of the side effects.
Do not be alarmed by the following list of side effects. You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor or pharmacist if you notice any of the following and they worry you:
- unpleasant taste
- nosebleeds
- nasal discomfort
- headache
The above list includes the more common side effects of your medicine.
Tell your doctor as soon as possible if you notice any of the following:
- Sleepiness or drowsiness
- Nasal problems, which may include:
- crusting in the nose
- nosebleeds
- hole in the cartilage between your nose (nasal septal perforation). A whistling sound when you breathe may be a symptom of nasal septal perforation. - Slow wound healing. You should not use RYALTRIS® until your nose has healed if you have a sore in your nose, if you have had surgery on your nose, or if your nose has been injured.
- Thrush (Candida), a fungal infection in your nose and throat. Tell your healthcare provider if you have any redness or white-colored patches in your nose or mouth.
- Symptoms of infection which may include the following:
- fever
- aches or pains
- chills
- feeling tired - Symptoms of adrenal insufficiency which may include the following:
- tiredness
- weakness
- nausea
- vomiting
- low blood pressure - Slowed or delayed growth in children. RYALTRIS® should not be given to children under the age of 6 years. A child’s growth should be checked regularly while using intranasal corticosteroids.
If you develop a rash, wheezing or breathlessness, stop using the nasal spray, as you may be allergic to it, and contact your doctor immediately.
Other side effects not listed above may also occur in some patients. Tell your doctor if you notice anything that is making you feel unwell.
Long-term use of corticosteroid sprays may be associated with other side effects. Your doctor will monitor your health if you are using RYALTRIS® for extended periods of time.
After using RYALTRIS®
Storage
Keep the nasal spray in a cool dry place where the temperature stays below 30°C. Do not freeze or refrigerate. Store it away from heat.
Do not store it or any other medicine in the bathroom or near a sink. Do not leave it in the car or on windowsills. Heat and dampness can destroy some medicines.
Keep it where children cannot reach.
Disposal
If your doctor tells you to stop using this medicine or it has passed the expiry date, ask your pharmacist what to do with any that is left over.
Further information
Drug testing for sport events:
This product contains a corticosteroid for intranasal administration; it is not a restricted drug for sports.
Corticosteroids may be detected in blood and in the urine during drug testing; thus prior written permission for its use may be required by some sport agencies.
Product description
What it looks like
The nasal spray is a white liquid suspension contained in a plastic bottle with a manual spray pump fitted with a white actuator and purple cap for intranasal administration.
Ingredients
Active ingredients:
Olopatadine hydrochloride
Mometasone furoate monohydrate
Inactive ingredients:
Carmellose sodium
Dibasic sodium phosphate heptahydrate
Disodium edetate
Dispersible cellulose
Hydrochloric acid
Polysorbate 80
Sodium chloride
Sodium hydroxide
Water for injection
Preservative:
Benzalkonium chloride
Sponsor
Seqirus Pty Ltd
ABN: 26 160 735 035
63 Poplar Road
Parkville VIC 3052
Australia
Telephone: 1800 642 865
www.seqirus.com.au
Australian Registration Number
1 x 240 metered dose bottle per pack
1 x 120 metered dose bottle per pack
1 x 56 metered dose bottle per pack
AUST R 312690
Date of Preparation
February 2023
RYALTRIS® is a registered trade mark of Glenmark Specialty SA.
Published by MIMS August 2023