What is in this leaflet
Please read this leaflet carefully before you use your medicine.
This leaflet answers some common questions about Salbutamol Cipla cipules. It does not contain all the available information
It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking Salbutamol Inhalation Solution against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine. You may need to read it again.
What Salbutamol Cipla cipules are used for
Salbutamol Cipla Cipules contains the active substance, salbutamol which is known as a bronchodilator. It helps you breathe more easily. When your chest is tight or when you are wheezing, Salbutamol Cipla Cipules opens up the breathing tubes in your lungs.
Because the medicine in your Salbutamol Cipla Cipules gives fast relief from your chest symptoms, it is often called a 'reliever'. There are other types of medicines that prevent wheezing or chest tightness. These medicines are called 'preventers' and must be used every day. Your doctor may tell you to use a 'preventer' in addition to your Salbutamol Cipla Cipules.
Salbutamol inhalation solution are not the only form of salbutamol available. Your doctor will decide which form of salbutamol is right for you.
Ask your doctor if you have any questions about why this medicine has been prescribed for you. Your doctor may have prescribed it for another reason.
Before you use Salbutamol Cipla cipules
When you must not be given it
Do not use Salbutamol Inhalation Solution if you have an allergy to salbutamol sulfate or any other ingredients in Salbutamol Cipla Cipules (see Ingredients)
Do not take Salbutamol Cipla Cipules to stop a miscarriage or premature labour.
Do not take this medicine after the expiry date printed on the pack has passed or if the packaging is torn or shows signs of tampering. If it has expired or if the packaging is damaged, return it to your pharmacist for disposal.
If you are not sure whether you should start taking this medicine, talk to your doctor.
Before you start to take it
You must tell your doctor if:
- you are allergic to foods, dyes, preservatives or any other medicines.
- you have had to stop taking this or any other asthma medicine for any reason
- you have been diagnosed with a thyroid condition
- you have been diagnosed with high blood pressure
- you have a heart condition
- you have a liver condition
- you have a kidney condition
- you have diabetes
Tell your doctor if you are pregnant or plan to become pregnant or are breast- feeding. The ingredients of Salbutamol Cipla Cipules are known to cross the placenta during pregnancy. Your doctor can discuss with you the risks and benefits involved.
It is not known whether the ingredients of Salbutamol Cipla Cipules can pass into breast milk. If you are breast- feeding, you must check with your doctor before you take Salbutamol Cipla Cipules.
If you have not told your doctor about any of the above, tell him/ her before you start taking Salbutamol Cipla Cipules.
Taking other medicines
Tell your doctor or pharmacist if you are taking any other medicines, including any that you buy without a prescription from your pharmacy, supermarket or health food shop.
Some medicines may interfere with salbutamol. Your doctor and pharmacist have more information on medicines to be careful with or avoid while taking this medicine.
How to take Salbutamol Cipla cipules properly
Follow all directions given to you by your doctor or pharmacist carefully.
They may differ from the information contained in this leaflet.
If you do not understand the instructions on the box, ask your doctor or pharmacist for help.
How much to take
Take Salbutamol Cipla Cipules as directed by your doctor or pharmacist. The pharmacist's label will usually tell you how many Salbutamol Cipla Cipules should be taken. If you are not sure, ask your doctor or pharmacist.
Adults: one 5 mg cipule via nebuliser every 4-6 hours as necessary.
Children (4 -12 years): one 2.5 mg cipule via nebuliser every 4-6 hours as necessary.
Initial doses in the elderly may be lower than the recommended adult dose.
If your condition suddenly gets worse, your doctor may tell you to increase your dose.
How to take it
Salbutamol Cipla Cipules must only be used by inhalation from a nebuliser and must not be injected or swallowed.
The nebuliser produces a fine mist which you breathe in through a face mask or a mouthpiece. Make sure you know how to use it properly. If you have problems ask your doctor or pharmacist.
Do not let the liquid, or the mist produced by the nebuliser get into your eyes. You can wear glasses or goggles to protect them.
Use your nebuliser in a well ventilated room as some of the mist will be released into the air and may be breathed in by others
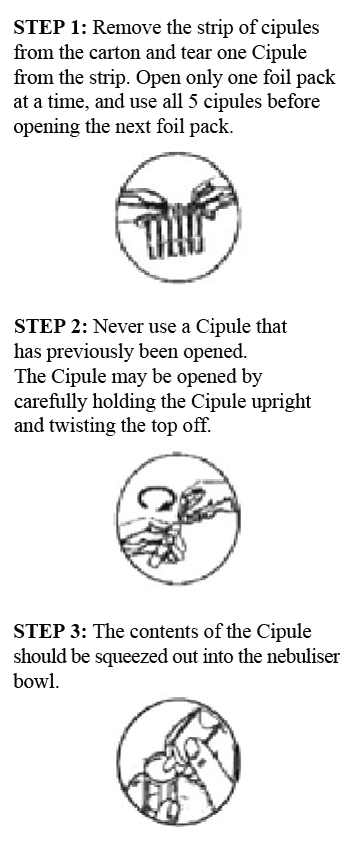
The nebuliser should be assembled and used as directed by your doctor. Any solution remaining in the nebuliser should be discarded after use. Follow the manufacturer's instructions on how to clean your nebuliser.
How long to take it
Your doctor will decide how often and for how long you have to take Salbutamol Cipla Cipules. The pharmacist's label will usually tell you how often to take Salbutamol Cipla Cipules. If you are not sure, ask your doctor or pharmacist.
Do not stop taking Salbutamol Cipla Cipules, or change the dose without first checking with your doctor.
If you forget to take it
Do not take an extra dose to make up for a missed dose.
This may increase the chance of you getting unwanted side effects.
Just take your next dose at the usual time. If you become wheezy or develop other symptoms of an asthma attack you may need to take your next dose earlier.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering to take your medicine, ask your pharmacist for some hints.
If you take too much (overdose)
Immediately telephone your doctor or the Poisons Information Centre (In Australia call 13 11 26) for advice, or go to Accident and Emergency at the nearest hospital, if you think that you or anyone else may have taken too many Salbutamol Cipla Cipules. Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
Symptoms of an overdose may include:
- rapid heart beat
- significant muscle tremor
- increased acid in the blood, which may cause an increased rate of breathing.
While you are using Salbutamol inhalation
Things you must do
If you are about to be started on any new medicine, remind your doctor or pharmacist that you are taking Salbutamol Cipla Cipules.
Tell any other doctors, dentists, and pharmacists who treat you that you are taking this medicine.
If you are going to have surgery, tell the surgeon or anaesthetist that you are taking this medicine. It may affect other medicines used during surgery.
If you become pregnant while taking this medicine, tell your doctor immediately.
If you are about to have any blood tests, tell your doctor that you are taking this medicine. It may interfere with the results of some tests.
Keep all of your doctor's appointments so that your progress can be checked.
If your Salbutamol Cipla Cipules do not help your breathing as much as usual, tell your doctor as soon as possible.
If the effect of your Salbutamol Cipla Cipules does not last as long as usual, tell your doctor as soon as possible. These may be signs that your chest condition is getting worse.
If your Salbutamol Cipla Cipules are not having the same effect as before, your doctor may decide to prescribe other forms of salbutamol, or another medicine, for you to use.
This medicine is only one part of a general plan to help you manage your asthma or other chest condition. You should discuss this plan with your doctor. Ask your doctor to check your treatment regularly.
Things you must not do
Do not take salbutamol to treat any other complaints unless your doctor tells you to.
Do not give your medicine to anyone else, even if they have the same condition as you.
Do not stop taking your medicine or lower the dosage without checking with your doctor. If you stop taking it suddenly, your condition may worsen or you may have unwanted side effects.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking Salbutamol Cipla cipules.
Like other medicines Salbutamol Cipla cipules may have unwanted side effects in a few people. Sometimes they are serious, most of the time they are not. You may need medical attention if you get some of the side effects.
Do not be alarmed by the following lists of side effects. You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor or pharmacist if you notice any of the following side effects and they worry you.
Common side effects:
- headache
- nausea
- shaky or tense feeling
- irregular or fast heart beat
- 'warm' feeling or flushing
Rare side effects:
- mouth and throat irritation
- muscle cramps
- restlessness in children.
Very rare side effects:
- feeling unusually active, such as restlessness and excitability.
If your breathing or wheezing gets worse straight after taking this medicine, stop using it immediately, and tell your doctor immediately. If available, try a different fast-acting asthma medicine as soon as possible.
If any of the following happen, tell your doctor immediately or go to Accident and Emergency at your nearest hospital. Allergic reactions have been very rarely reported. These symptoms may include a skin rash, angioedema (sudden swelling under the skin) or a faint or dizzy feeling.
Other side effects not listed above may also occur in some people.
Some of these side effects such as changes to blood potassium levels have been seen rarely and can only be found when your doctor does tests from time to time to check your progress.
After using Salbutamol Cipla Cipules
Storage
Keep your medicine in the pack until it is time to take them. If you take the medicine out of the pack they may not keep well.
Salbutamol Cipla Cipules should be kept in a cool, dry place where the temperature stays below25°C. Cipule must be protected from light.
Do not store in direct sunlight or heat. Do not leave in the car on hot days.
Heat and dampness can destroy some medicines.
Keep it where children cannot reach it.
Do not use after the expiry date on the carton.
Once you have opened each foil pack, you need to note down the date of opening the foil lid. Add three months to this date and write it down in the space provided on the foil pack. Do not use the Salbutamol Cipla Cipules left in the tray after this date.
A locked cupboard at least one-and-a half metres above the ground is a good place to store medicines. Do not store Salbutamol Cipla Cipules or any other medicine in the bathroom or near a sink.
Disposal
If your doctor tells you to stop using Salbutamol Cipla or it has passed its expiry date, ask your pharmacist what to do with any that are left over.
Product description
What it looks like
Salbutamol Cipla Cipules contain salbutamol solution for inhalation. It is a clear colourless, preservative free solution and comes in plastic unit dose ampoules.
Salbutamol Cipla Cipules comes in two strengths.
AUST R 115652 2.5mg Salbutamol Sulphate in 2.5ml of solution, 30 (6X5)
AUSTR 115657 5 mg Salbutamol Sulphate in 2.5ml of solution, 30(6X5)
Ingredients
Cipules contains the active ingredient Salbutamol Sulfate BP.
Your medicine also contains sodium chloride and water.
The solution is sterile and preservative free.
Supplier
Cipla Australia Ply Ltd
Level 1, 132-136 Albert Road
South Melbourne, VIC 3205
drugsafety@Cipla.com
Phone: 1 800 569 074.
This leaflet was prepared on Mar 2021.
Published by MIMS May 2021

 Chemical name: di[(RS)-2-(1,1-dimethyl)ethylarnino-1- [4-hydroxy-3 -(hydroxymethyl)phenyl]ethanol] sulfate.
Chemical name: di[(RS)-2-(1,1-dimethyl)ethylarnino-1- [4-hydroxy-3 -(hydroxymethyl)phenyl]ethanol] sulfate.