SAMSCA®
| Consumer Medicine Information (CMI) summary |
The full CMI on the next page has more details. If you are worried about using this medicine, speak to your doctor or pharmacist.
| 1. Why am I using SAMSCA? |
SAMSCA contains the active ingredient tolvaptan. SAMSCA is used to treat hyponatraemia in adults, including patients who have conditions such as heart failure, and “syndrome of inappropriate antidiuretic hormone secretion” (SIADH).
For more information, see Section 1. Why am I using SAMSCA? in the full CMI.
| 2. What should I know before I take SAMSCA? |
Do not use if you have ever had an allergic reaction to SAMSCA or any of the ingredients listed at the end of the CMI.
Talk to your doctor if you have any other medical conditions, take any other medicines, or are pregnant or plan to become pregnant or are breastfeeding.
For more information, see Section 2. What should I know before I take SAMSCA? in the full CMI.
| 3. What if I am taking other medicines? |
Some medicines may interfere with SAMSCA and affect how it works.
A list of these medicines is in Section 3. What if I am taking other medicines? in the full CMI.
| 4. How do I take SAMSCA? |
- Take SAMSCA once a day with or without food
- The dose can be from 15 mg to 60 mg once a day
More instructions can be found in Section 4. How do I take SAMSCA? in the full CMI.
| 5. What should I know while taking SAMSCA? |
| Things you should do |
|
| Things you should not do |
|
| Driving or using machines |
|
| Drinking alcohol |
|
| Looking after your medicine |
|
For more information, see Section 5. What should I know while taking SAMSCA? in the full CMI.
| 6. Are there any side effects? |
Common side effects are thirst; dry mouth; making large amounts of urine and urinating often. Serious side effects include difficulty swallowing and speaking; seizures or convulsions; vomiting bright red blood; vomiting dark blood clots, or materials that looks like coffee-grounds; passing blood or stool mixed with blood; lethargy/fatigue (tiredness); mood changes; difficulty moving arms or legs, or uncontrollable movements.
For more information, including what to do if you have any side effects, see Section 6. Are there any side effects? in the full CMI.
SAMSCA®
Active ingredient(s): tolvaptan (tol-vap-tan)
| Consumer Medicine Information (CMI) |
This leaflet provides important information about using SAMSCA. You should also speak to your doctor or pharmacist if you would like further information or if you have any concerns or questions about using SAMSCA.
Where to find information in this leaflet:
1. Why am I using SAMSCA?
2. What should I know before I take SAMSCA?
3. What if I am taking other medicines?
4. How do I take SAMSCA?
5. What should I know while taking SAMSCA?
6. Are there any side effects?
7. Product details
| 1. Why am I using SAMSCA? |
SAMSCA contains the active ingredient tolvaptan.
SAMSCA belongs to a group of medicines called vasopressin antagonists. This means that it prevents vasopressin having its effect on water retention. This leads to a reduction in the amount of water in the body by increasing urine production and as a result it increases the concentration of sodium in your blood.
SAMSCA is used to treat hyponatraemia in adults, including patients who have conditions such as heart failure, and “syndrome of inappropriate antidiuretic hormone secretion” (SIADH). These conditions result in an inappropriate amount of the hormone vasopressin causing the sodium levels in your blood to get too low (hyponatraemia). That can lead to difficulties in concentration and memory, or in keeping your balance.
| 2. What should I know before I take SAMSCA? |
Warnings
Do not take SAMSCA if:
- you are allergic to tolvaptan, any of the ingredients listed at the end of this leaflet, or any other similar medicine such as benzapine derivatives
- Always check the ingredients to make sure you can use this medicine.
- some of the symptoms of an allergic reaction may include:
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body
- rash, itching or hives on the skin - have any of the following medical conditions:
- you cannot replace fluids by drinking
- you cannot feel if you are thirsty
- you have a condition which is associated with a very low blood volume
- you are not producing or passing urine - you are taking the following medicine(s):
- medicines to treat fungal infections such as ketoconazole or itraconazole
- medicines to treat bacterial infections such as clarithromycin or telithromycin
- medicines to treat HIV infection such as saquinavir, nelfinavir or ritonavir
- medicines to treat depression i.e. nefazodone - you need to increase the sodium level in your blood right away - the safety and efficacy in these patients have not been studied
Check with your doctor if you:
- have any other medical conditions such as:
- you cannot drink enough water or you are fluid restricted
- difficulties in urination or have an enlarged prostate
- liver disease including cirrhosis
- diabetes
- hypothyroidism (an underactive thyroid gland)
- hypoadrenalism (an underactive adrenal gland) - take any medicines for any other condition
During treatment, you may be at risk of developing certain side effects. It is important you understand these risks and how to monitor for them. See additional information under Section 6. Are there any side effects?
Pregnancy and breastfeeding
Check with your doctor if you are pregnant or intend to become pregnant.
Talk to your doctor if you are breastfeeding or intend to breastfeed.
It is not known if SAMSCA will harm your unborn baby or if it passes into breast milk. This medicine contains sugars as lactose.
If you have been told by your doctor that you have an intolerance to some sugars, tell your doctor before taking SAMSCA.
| 3. What if I am taking other medicines? |
Tell your doctor or pharmacist if you are taking any other medicines, including any medicines, vitamins or supplements that you buy without a prescription from your pharmacy, supermarket or health food shop.
Some medicines may interfere with SAMSCA and affect how it works. These include:
- medicines to treat fungal infections such as ketoconazole, itraconazole or fluconazole
- macrolide antibiotics such as clarithromycin or telithromycin or erythromycin
- treatments for high blood pressure and chest pain such as diltiazem or verapamil
- treatments of irregularities of heart beat and heart failure such as digoxin
- treatments for epilepsy/seizures and some sleep disorders such as barbiturates, phenytoin or carbamazepine
- medicines to treat tuberculosis such as rifampicin rifabutin or rifapentin
- medicines to immunosuppressant therapy such as cyclosporine
- treatments used to prevent or control bleeding, i.e. vasopressin analogs
- treatments of HIV infection such as saquinavir, nelfinavir or ritonavir; and
- medicines used to treat depression, i.e. nefazodone or St. John's Wort
- other medicines that increase the level of sodium in your blood or which contain large amounts of salt, such as tablets that dissolve in water and indigestion remedies
These medicines may be affected by SAMSCA or may affect how well it works. You may need different amounts of your medicines, or you may need to take different medicines.
Your doctor will have more information on medicines to be careful with or avoid while taking SAMSCA.
Check with your doctor or pharmacist if you are not sure about what medicines, vitamins or supplements you are taking and if these affect SAMSCA.
| 4. How do I take SAMSCA? |
Treatment with SAMSCA should be started and re-started only in a hospital, where the sodium levels in your blood can be monitored closely.
If your doctor or pharmacist tells you to keep taking SAMSCA after you leave a hospital, it is important that you do not stop and restart SAMSCA on your own.
You may need to go back to a hospital to re-start SAMSCA. Talk to your doctor right away if you stop taking SAMSCA for any reason.
It is important to stay under the care of your doctor while taking SAMSCA and follow their instructions.
If you do not understand the instructions, ask your doctor or pharmacist for help.
How much to take
- Take SAMSCA once a day
- You can take SAMSCA with or without food
- Tablets should be swallowed without chewing and with a glass of water
- Do not take SAMSCA with grapefruit juice
- The dose can be from 15 mg to 60 mg once a day. Your doctor will start with a dose of 15 mg and may then increase it to a maximum of 60 mg to achieve the desired level of serum sodium.
- Ask your doctor of pharmacist if you are unsure of the correct dose for you, they will tell you exactly how much to take
- Follow the instructions provided and use SAMSCA until your doctor tells you to stop
- SAMSCA should not be taken for longer than 30 days
- If you take the wrong dose, SAMSCA may not work as well and your problem may not improve
- Do not take more than the dose your doctor has recommended
When to take SAMSCA
- SAMSCA should be taken the same time each day, preferably in the morning
- This will help you remember when to take the medicine
- Certain medicines or illnesses may keep you from drinking fluids or may cause you to lose too much body fluid, such as vomiting or diarrhoea. If you have these problems, call your doctor right away.
If you forget to take SAMSCA
SAMSCA should be used regularly at the same time each day. If you miss your dose at the usual time, take it as soon as you remember and then go back to taking your medicine as you would normally.
If it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to.
Do not take a double dose to make up for the dose you missed.
- This may increase the chance of you getting an unwanted side effect
If you are not sure what to do, ask your doctor or pharmacist
If you have trouble remembering to take your medicine, ask your pharmacist for some hints.
If you take too much SAMSCA
If you think that you have taken too much SAMSCA, you may need urgent medical attention.
You should immediately:
- phone the Poisons Information Centre
(by calling 13 11 26), or - contact your doctor, or
- go to the Emergency Department at your nearest hospital.
You should do this even if there are no signs of discomfort or poisoning.
You may need urgent medical attention. Remember to take the medicine pack with you so that it is clear what you have taken.
Symptoms of an overdose may include:
- thirst
- dehydration
- passing more urine than normal
- low blood volume causing very low blood pressure
- a rise in concentration of sodium concentration the blood
If your doctor tells you to stop taking SAMSCA, follow their instructions about limiting the amount of fluid you should drink.
| 5. What should I know while taking SAMSCA? |
Things you should do
Make sure you have access to water and continue to drink when thirsty.
If you are about to be started on any new medicine, remind your doctor and pharmacist that you are taking SAMSCA.
If you are going to have surgery, tell the surgeon or anaesthetist that you are taking this medicine.
It may affect other medicines used during surgery.
If you are about to have any blood tests, tell your doctor that you are taking this medicine.
It may interfere with the results of some tests.
Call your doctor straight away if you:
- become pregnant while taking this medicine
Remind any doctor, dentist or pharmacist you visit that you are using SAMSCA.
Things you should not do
- Do not use SAMSCA to treat any complaint other than that directed by your doctor
- Do not give SAMSCA to someone else even if their symptoms are the same
- Do not stop taking SAMSCA or lower the dose unless you notice side effects requiring urgent medical attention or if your doctor tells you to do so. This may lead to reoccurrence of your low sodium.
Drinking enough water
- SAMSCA causes water loss because it increases your urine production. This water loss commonly results in dry mouth and thirst, and less commonly severe side effects such as kidney problems. It is therefore important that you have access to water and that you are able to drink sufficient amounts when you feel thirsty.
Driving or using machines
Be careful before you drive or use any machines or tools until you know how SAMSCA affects you.
SAMSCA may occasionally make you feel dizzy or weak or you may faint for a short period.
Drinking alcohol
Tell your doctor if you drink alcohol.
Looking after your medicine
- Store SAMSCA below 25°C and away from light and moisture.
- Do not leave it in the car on hot or cold days. Heat, cold and dampness can destroy some medicines.
Follow the instructions in the carton on how to take care of your medicine properly.
Store it in a cool dry place away from moisture, heat or sunlight; for example, do not store it:
- in the bathroom or near a sink, or
- in the car or on window sills.
Keep it where young children cannot reach it.
A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Keep SAMSCA in the pack until it is time to take your dose. If it is removed from the pack, it may not keep well.
Getting rid of any unwanted medicine
If your doctor tells you to stop taking SAMSCA or it is out of date, ask your doctor what to do with any SAMSCA that is left.
Do not use this medicine after the expiry date.
| 6. Are there any side effects? |
All medicines can have side effects. If you do experience any side effects, most of them are minor and temporary. However, some side effects may need medical attention.
See the information below and, if you need to, ask your doctor or pharmacist if you have any further questions about side effects.
Less serious side effects
| Less serious side effects | What to do |
General well-being related:
| Speak to your doctor if you have any of these less serious side effects and they worry you. |
| Less serious side effects | What to do |
Gastrointestinal related
| Speak to your doctor if you have any of these less serious side effects and they worry you. |
Serious side effects
| Serious side effects | What to do |
General well-being related:
| Call your doctor straight away, or go straight to the Emergency Department at your nearest hospital if you notice any of these serious side effects. |
SAMSCA may affect your liver function. Therefore, please inform your doctor at any time if you have signs that could indicate potential liver problems such as:
- unusual exhaustion
- loss of appetite
- pain in your right upper stomach
- dark urine
- jaundice (yellowing of your skin or the whites of your eyes)
During treatment with SAMSCA, your doctor may arrange blood tests to check for changes in your liver function or electrolytes (salts).
Tell your doctor or pharmacist if you notice anything else that may be making you feel unwell.
Other side effects not listed here may occur in some people.
Reporting side effects
After you have received medical advice for any side effects you experience, you can report side effects to the Therapeutic Goods Administration online at www.tga.gov.au/reporting-problems. By reporting side effects, you can help provide more information on the safety of this medicine.
Always make sure you speak to your doctor or pharmacist before you decide to stop taking any of your medicines.
| 7. Product details |
This medicine is only available with a doctor's prescription.
What SAMSCA contains
| Active ingredient (main ingredient) |
|
| Other ingredients (inactive ingredients) |
|
| Potential allergens | sugars as lactose |
SAMSCA tablets contain 15 mg of tolvaptan.
Do not take this medicine if you are allergic to any of these ingredients.
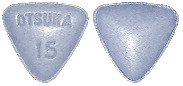
What SAMSCA looks like
SAMSCA is supplied as 15 mg tablets in a PVC/aluminium perforated unit dose blister pack of 10 tablets.
The 15 mg tablet is blue, triangular, shallow-convex, debossed with “OTSUKA” and “15” on one side.
(Aust R 176602).
Who distributes SAMSCA
Otsuka Australia Pharmaceutical Pty Ltd
Suite 2.03, Level 2
9 Help Street
Chatswood NSW 2067
This leaflet was prepared in June 2022.
SAMSCA® is a registered trademark of Otsuka Pharmaceutical Co., Ltd.
Published by MIMS August 2022

 In a subgroup of patients with hyponatraemia (N = 475, serum sodium < 135 mmol/L) enrolled in a double blind, placebo controlled trial (mean duration of treatment was 9 months) of patients with worsening heart failure, the following adverse reactions occurred in tolvaptan treated patients at a rate at least 2% greater than placebo: mortality (42% tolvaptan, 38% placebo), nausea (21% tolvaptan, 16% placebo), thirst (12% tolvaptan, 2% placebo), dry mouth (7% tolvaptan, 2% placebo) and polyuria or pollakiuria (4% tolvaptan, 1% placebo).
In a subgroup of patients with hyponatraemia (N = 475, serum sodium < 135 mmol/L) enrolled in a double blind, placebo controlled trial (mean duration of treatment was 9 months) of patients with worsening heart failure, the following adverse reactions occurred in tolvaptan treated patients at a rate at least 2% greater than placebo: mortality (42% tolvaptan, 38% placebo), nausea (21% tolvaptan, 16% placebo), thirst (12% tolvaptan, 2% placebo), dry mouth (7% tolvaptan, 2% placebo) and polyuria or pollakiuria (4% tolvaptan, 1% placebo). In patients with hyponatraemia (defined as < 135 mmol/L), serum sodium concentration increased to a significantly greater degree in tolvaptan treated patients compared to placebo treated patients as early as 8 hours after the first dose, and the change was maintained for 30 days. The percentage of patients requiring fluid restriction (defined as ≤ 1 L/day at any time during the treatment period) was also significantly less (p < 0.01) in the tolvaptan treated group (30/215, 14%) as compared with the placebo treated group (51/206, 25%).
In patients with hyponatraemia (defined as < 135 mmol/L), serum sodium concentration increased to a significantly greater degree in tolvaptan treated patients compared to placebo treated patients as early as 8 hours after the first dose, and the change was maintained for 30 days. The percentage of patients requiring fluid restriction (defined as ≤ 1 L/day at any time during the treatment period) was also significantly less (p < 0.01) in the tolvaptan treated group (30/215, 14%) as compared with the placebo treated group (51/206, 25%). See Figure 2.
See Figure 2. In the open label study SALTWATER, 111 patients, 94 of them hyponatraemic (serum sodium < 135 mmol/L), previously on tolvaptan or placebo therapy were given Samsca as a titrated regimen (15 to 60 mg once daily) after having returned to standard care for at least 7 days. By this time, their baseline mean serum sodium concentration had fallen to between their original baseline and postplacebo therapy level. Upon initiation of therapy, average serum sodium concentrations increased to approximately the same levels as observed for those previously treated with tolvaptan, and were sustained for at least a year. Figure 3 shows results from 111 patients enrolled in the SALTWATER study.
In the open label study SALTWATER, 111 patients, 94 of them hyponatraemic (serum sodium < 135 mmol/L), previously on tolvaptan or placebo therapy were given Samsca as a titrated regimen (15 to 60 mg once daily) after having returned to standard care for at least 7 days. By this time, their baseline mean serum sodium concentration had fallen to between their original baseline and postplacebo therapy level. Upon initiation of therapy, average serum sodium concentrations increased to approximately the same levels as observed for those previously treated with tolvaptan, and were sustained for at least a year. Figure 3 shows results from 111 patients enrolled in the SALTWATER study.
 Tolvaptan is practically insoluble in water and the aqueous solubility of the drug substance is poor (~ 0.1 mg/250 mL) across all pH ranges. It is slightly soluble in ethyl acetate, sparingly soluble in ethanol, soluble in methanol and freely soluble in benzyl alcohol. The octanol:water partition coefficient was reported to be greater than 5000 at 25°C.
Tolvaptan is practically insoluble in water and the aqueous solubility of the drug substance is poor (~ 0.1 mg/250 mL) across all pH ranges. It is slightly soluble in ethyl acetate, sparingly soluble in ethanol, soluble in methanol and freely soluble in benzyl alcohol. The octanol:water partition coefficient was reported to be greater than 5000 at 25°C.