What is in this leaflet
This leaflet answers some common questions about Semglee.
It does not contain all the available information. It does not take the place of talking to your doctor, pharmacist or diabetes educator.
All medicines have risks and benefits. Your doctor has weighed the risks of you using Semglee against the benefits they expect it will have for you.
If you have any concerns about using this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine. You may need to read it again
What Semglee is used for
Semglee is used to reduce high blood sugar (glucose) levels in people with diabetes mellitus.
Semglee is a modified insulin that is very similar to human insulin. It is a substitute for the insulin produced by the pancreas.
Semglee is a long-acting insulin. Other names for this insulin include insulin glargine and Lantus. Your doctor may tell you to use a rapid- acting human insulin or oral diabetes medication in combination with Semglee.
Semglee is not addictive.
Ask your doctor if you have any questions about why Semglee has been prescribed for you.
Before you use Semglee
When you must not use Semglee
Do not use Semglee:
If you have an allergy to:
- any medicine containing insulin
- any of the ingredients contained in Semglee listed at the end of this leaflet
Some of the symptoms of an allergic reaction may include:
- redness, swelling, rash and itching at the injection site
- rash, itching or hives on the skin
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body
If you are experiencing low blood sugar levels (hypoglycaemia - a "hypo"). If you have a lot of hypos discuss appropriate treatment with your doctor.
After the expiry date printed on the pack or if the packaging is torn or shows signs of tampering. If you use Semglee after the expiry date has passed, it may not work as well. If it has expired or is damaged, return it to your pharmacist for disposal.
If the product appears cloudy, discoloured or contains particles, or if the injection pen appears damaged.
If you are not sure whether you should start using this medicine, talk to your doctor.
Do not give Semglee to children less than 6 years of age. There is no experience with the use of Semglee in children less than 6 years.
Before you start to use Semglee
Tell your doctor if you have allergies to any other medicines, foods, preservatives or dyes.
Tell your doctor if you have or have had any of the following medical conditions:
- kidney problems
- liver problems
Tell your doctor if you are pregnant or plan to become pregnant. Pregnancy may make managing your diabetes more difficult.
Tell your doctor if you are breastfeeding or plan to breastfeed.
Tell your doctor if:
- you drink alcohol
- you do not eat regular meals
- you do a lot of exercise
- you are ill or feeling unwell
Alcohol, diet, exercise and your general health all affect the control of your diabetes.
Taking other medicines
Tell your doctor if you are taking any other medicines, including any that you get without a prescription from your pharmacy, supermarket or health food shop.
Medicines that may increase the blood sugar lowering effect of Semglee include:
- oral antidiabetic medicines that are used to treat type 2 diabetes
- blood pressure, blood flow, cholesterol and heart medications
- medications for pain and inflammation
- some antidepressants
- sulfonamide antibiotics
Medicines that may reduce the blood sugar lowering effect of Semglee include:
- corticosteroids, glucagon and other hormonal therapies
- estrogens, progestogens, oral contraceptives and gynaecological medications
- fluid and glaucoma medications
- tuberculosis and HIV/AIDS treatments
- some psychiatric medications
- adrenaline (epinephrine) and asthma medications such as salbutamol, terbutaline
Certain heart medications, especially beta-blockers, may mask the symptoms of hypoglycaemia.
Your doctor and pharmacist have a full list of medicines with which you must be careful or avoid while using Semglee. Please check with your doctor or pharmacist before starting any new medicines or over the counter products.
How to use Semglee
Semglee is a clear solution that does not require shaking before use.
Your doctor, pharmacist or diabetes educator will have shown you how to use Semglee and what injection site to use.
Carefully follow all the directions.
Do not dilute Semglee.
Do not mix Semglee with any other insulin or solution.
Do not inject Semglee into a muscle or vein.
Semglee is intended for injection under the skin. It can be injected at any time during the day, however, at the same time every day.
Any change in this medicine should be made cautiously and only under medical supervision.
If you do not understand the instructions, ask your doctor, pharmacist or diabetes educator for help.
How much to use
Your doctor will tell you how much Semglee you need to use each day. Your doctor may increase or decrease the dose, depending on your blood sugar levels.
It is very important that you manage your diabetes carefully. Too much or too little insulin can cause serious effects.
When to use Semglee
Your doctor will tell you when to use Semglee.
Semglee should be used once a day, at the same time every day.
How to use Semglee injection pens
Wash your hands with soap and water before using your injection pen.
Always do a safety test before use. The safety test may highlight a problem with your injection pen. The safety test also removes any air bubbles and helps indicate whether or not a needle is blocked, bent or broken.
Never use an injection pen if it is damaged or you are not sure that it is working properly. Use a new pen.
Pre-filled disposable pens
Semglee disposable pens are pre- filled and ready to use. Do not attempt to replace the cartridge in a pre-filled disposable pen. Once all the insulin is used you cannot replace the cartridge. Empty disposable pens must never be reused and must be properly discarded
Keep the injection pen at room temperature for 1 or 2 hours before use. Cold insulin is more painful to inject.
Carefully follow the instructions provided with the Semglee pen for attaching a needle, performing a safety test and administering the insulin injection.
Becton Dickinson (BD Ultra-Fine™ +) or Novofine needles should be used with the injection pens.
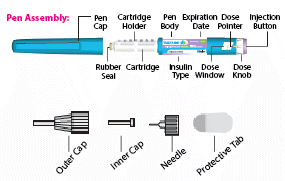
Guide to parts
INJECTING A DOSE
Step 1: Prepare your pen
- (A) Inspect the pen. Always check the insulin label on the reusable pen before each injection to make sure:
- It is the correct injection pen
- It is the correct insulin type
- The expiry date has not passed - (B) Hold the pen body with one hand. With the other hand pull off the pen cap. Put the pen cap aside to be used later.
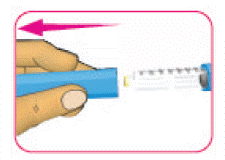
- (C) Check the insulin through the cartridge holder to make sure:
- The insulin looks clear and colourless with no visible particles
- There are no cracks, breaks or leaks around the cartridge holder - (D) Wipe the rubber seal (at the front of the cartridge) with a new alcohol wipe.
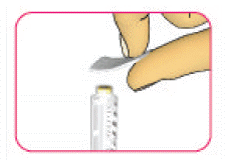
Step 2: Attach a new needle
- (A) Take a new sterile disposable needle and peel off the protective seal. Do not use the needle if the protective seal is damaged or missing as the needle may not be sterile.
- (B) While holding the pen body facing upwards, attach the outer needle cap straight on to the cartridge holder as shown. Trying to attach the outer needle cap sideways may bend or damage the needle.
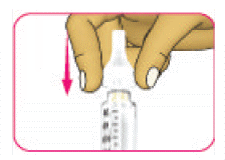
- (C) Turn the outer needle cap in a clockwise (right) direction until it feels tightly fixed on the pen.
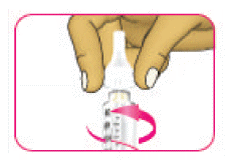
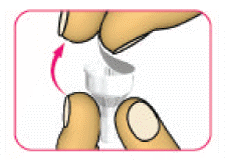
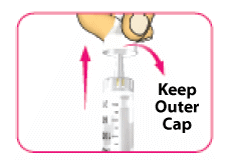
- (D) Carefully pull off the outer needle cap and put it aside. Do not throw it away. You will need the outer needle cap later.
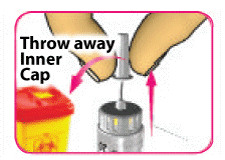
- (E) Carefully pull off the inner needle cap and throw it away.
Step 3: Do a safety test on your pen needle
- (A) Always do a safety test on a new pen needle before each injection.
- (B) Turn the white dose knob to 2 dose units. You will hear a "click" for each unit turned.
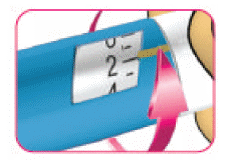
If you accidentally turn past 2 units, turn back the dose knob in the opposite direction to the correct number of units.
- (C) Hold the pen body facing upwards with one hand.
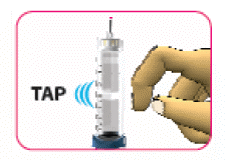
- (D) Tap the cartridge gently with your finger to help any large air bubbles to move to the top of the cartridge. Small bubbles may still be visible. This is normal.
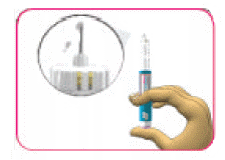
- (E) With the pen upright, press the injection button in until it stops moving and the dose window shows "0".
- (F) Repeat steps 3B through 3E up to four times until you see drops of insulin at the tip of the needle. The safety test is complete when you can see drops of insulin.
- If you do not see any insulin at the needle tip after 4 safety testing attempts the needle may be blocked. If this occurs:
- Go to Step 7 for instructions on safely removing the needle.
- Restart the process at step 2A to attach and do safety testing for a new needle.
Step 4: Select your dose
- (A) Check that the dose window shows "0".
- (B) Turn the white dose knob until the yellow dose pointer lines up with your required dose
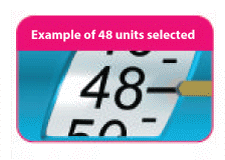
As you turn the white dose knob to set your dose, the white plunger will extend out and you will hear a "click" at each unit dialed.
The dose can be corrected by turning the dose knob in either direction until the correct dose lines up with the yellow dose pointer.
The pen will not let you dial a dose more than the number of units left in the Pen. If your dose is more than the number of units left in the Pen, either:
- Inject the amount left in your pen and use a new pen to give the rest of your dose,
Or
- Get a new pen and inject the full dose.
Do not force the dose knob to turn beyond 80 units.
Do not push the purple injection button when turning the dose knob.
Step 5: Select and clean the injection site
- (A) Select the injection site as explained to you by your doctor, pharmacist or diabetes educator, clean with a new alcohol wipe and let your skin dry before you inject your dose.
Injection sites include your arms, hips, thighs, buttocks and abdomen. You should change your injection site for each injection.
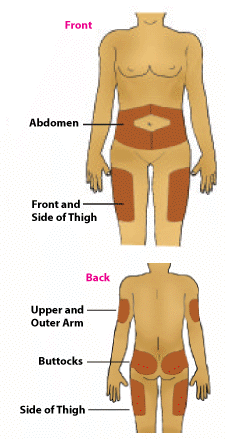
Use a different injection site each injection so that the same site is not used more often than once a month.
This will reduce the chance of local skin reactions developing.
Step 6: Inject your dose
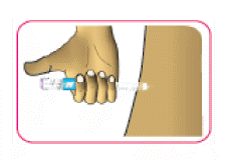
- (A) If instructed by your doctor, pharmacist or diabetes educator, you can stabilise the cleaned skin by spreading it or pinching up a large area between your fingers.
- (B) Push the needle straight into the skin as shown by your doctor, pharmacist or diabetes educator.
Do not inject with the needle at an angle
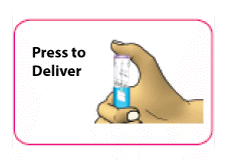
- (C) Press the purple injection button all the way in. The white dose knob will turn and you will hear "clicks" as you press down.
- (D) Hold the purple injection button down for 10 seconds after the dose window shows "0" to make sure all of the insulin is injected. If you do not keep the injection button pressed down for 10 seconds after "0" is displayed you may get the wrong dose of medicine.
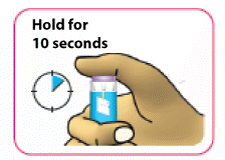
Do not push the injection button sideways or block the white dose knob with your fingers as this will stop you from injecting the medicine.
Step 7: After your injection
- (A) Take the outer needle cap that you had saved in step 2D, hold it at the widest part and carefully cover the needle without touching it.
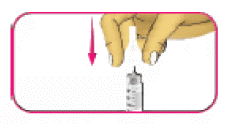
- (B) Squeeze the wide part of the outer needle cap and unscrew the needle in a counter-clockwise (left) direction. Keep twisting the needle until it comes off the pen. It may take several twists to release the needle.
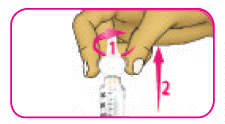
- (C) Dispose of the needle safely into a sharps container or as instructed by your healthcare professional.
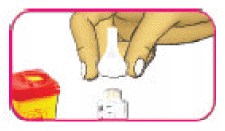
- (D) Replace the pen cap over the cartridge.
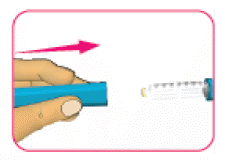
- (E) Store the pen at room temperature (below 30°C). Do not store the pen with a used needle attached
How long to use Semglee
Continue using Semglee for as long as your doctor recommends.
Make sure you keep enough Semglee to last over weekends and holidays.
Always carry an extra Semglee prefilled pen injector as recommended by your healthcare professional in case your pen gets lost or damaged.
If you use too much (overdose) - Hypoglycaemia, a "Hypo"
If you accidentally use too much Semglee your blood sugar level may become too low (hypoglycaemia).
Immediately telephone your doctor or the Poisons Information Centre (13 11 26 in Australia; 0800 POISON or 0800 764 766 in New Zealand) if you think that you or anyone else may have used too much Semglee. Do this even if there are no signs of discomfort or poisoning.
The risk of hypoglycaemia is increased if you:
- accidentally use too much Semglee
- have too much or unexpected exercise
- delay eating meals or snacks
- eat too little food
- are ill
The first symptoms of mild to moderate hypoglycaemia can come on suddenly. They may include:
- cold sweat, cool pale skin
- fatigue, drowsiness, unusual tiredness and weakness
- nervousness, anxious feeling, tremor, rapid heart beat
- confusion, difficulty concentrating
- excessive hunger
- vision changes
- headache, nausea
Always carry some sugary food or drink with you.
If you experience any of these symptoms of hypoglycaemia, you need to raise your blood sugar urgently. You can do this by taking one of the following:
- 5-7 jelly beans
- 3 teaspoons of sugar or honey
- 1/2 can of a sugar-containing soft drink (not a diet soft drink)
- 2-3 concentrated glucose tablets
Follow up with extra carbohydrates, e.g. plain biscuits, fruit or milk, when over the initial symptoms. Taking this extra carbohydrate will prevent a second drop in your blood sugar level.
If not treated quickly, the initial symptoms of hypoglycaemia may progress to loss of co-ordination, slurred speech, confusion, loss of consciousness and seizures.
If severe hypoglycaemia is not treated, it can cause brain damage and death.
Tell your relatives, friends, close workmates or carers that you have diabetes. It is important that they recognise the signs and symptoms of a "hypo".
Make sure they know to turn you on your side and get medical help immediately if you lose consciousness.
Make sure they know not to give you anything to eat or drink if you are unconscious. This is because you could choke.
Provide them with the telephone number for your doctor, the Poisons Information Centre (13 11 26 in Australia; 0800 POISON or 0800 764 766 in New Zealand) and Emergency Services.
An injection of the hormone glucagon may speed up recovery from unconsciousness. This can be given by a relative, friend, workmate or carer who knows how to give it.
If glucagon is used, have some sugary food or drink as soon as you are conscious again.
If you do not feel better after this, contact your doctor, diabetes educator, or the closest hospital.
If you do not respond to glucagon treatment, you will have to be treated in a hospital.
See your doctor if you keep having "hypos" or if you have ever become unconscious after using Semglee. Your dose of Semglee or other medicines may need to be changed.
If you miss a dose - Hyperglycaemia
If you forget to inject your insulin dose, test your blood sugar level as soon as possible.
Semglee is a long-acting insulin that works for 24 hours and should be injected regularly at the same time each day. If you miss injecting your dose at the regular scheduled time, your blood sugar levels may become high (hyperglycaemia).
However, injecting a dose of Semglee at another time may increase your risk of having a hypo. You should therefore plan in advance with your doctor or healthcare professional so that you know what to do in case you miss a dose.
If you have missed a dose and are not sure what you should do, contact your doctor or healthcare professional for specific advice.
Do NOT use a double dose of your insulin. If you double a dose, this may cause low blood sugar levels.
The risk of hyperglycaemia is increased if you:
- miss doses of Semglee or other insulin, or use less Semglee than you need
- have uncontrolled diabetes
- exercise less than usual
- eat more carbohydrates than usual
- are ill or stressed
- take certain other medications
High blood sugar levels over a period of time can lead to too much acid in the blood (diabetic ketoacidosis).
Contact your doctor immediately if your blood sugar level is very high or you experience any of the following symptoms.
Symptoms of mild to moderate hyperglycaemia include:
- drowsy feeling
- flushed face
- thirst, loss of appetite
- fruity odour on the breath
- blurred vision
- passing larger amounts of urine than usual
- getting up at night more often than usual to pass urine
- high levels of glucose and acetone in the urine
Symptoms of severe hyperglycaemia include:
- heavy breathing
- fast pulse
- nausea, vomiting
- dehydration
- loss of consciousness
Severe hyperglycaemia can lead to unconsciousness and, in extreme cases, death if untreated.
Discuss any worries you may have about this with your doctor, pharmacist or diabetes educator.
While you are using Semglee
Things you must do
Measure your blood sugar level regularly. This is the best way to tell if your diabetes is being controlled properly. Your doctor or diabetes educator will show you how and when to do this.
It is important to keep using Semglee even if you feel well. Semglee helps to control your condition, but does not cure it.
Tell your doctor if you often have hypoglycaemia or if you have ever become unconscious after using Semglee. Your doctor may need to adjust your dose of Semglee or of other medicines you are taking.
Always carry some sugary food or drink with you. If you experience any of the symptoms of hypoglycaemia, immediately eat some sugary food or have a drink, e.g. jelly beans, sugar, honey, sugar-containing soft drink, glucose tablets. Diet and low calorie soft drinks do NOT contain sugar and are unsuitable to take for hypoglycaemia.
Make sure that you tell every doctor, dentist, pharmacist or other healthcare professional who is treating you that you have diabetes and are using Semglee.
Tell your doctor, pharmacist or diabetes educator if you are travelling. Ask your doctor for a letter explaining why you are taking injecting pens and needles with you. Each country you visit will need to see this letter, so you should take several copies.
You may need to inject Semglee and eat your meals at different times because of time differences in and between countries.
If you are travelling, it is a good idea to:
- wear some form of identification showing you have diabetes
- carry some form of sugar to treat hypoglycaemia if it occurs, e.g. sugar sachets or jelly beans
- carry emergency food rations in case of a delay, e.g. dried fruit, biscuits or muesli bars
- keep Semglee readily available; take enough Semglee for your expected needs whilst travelling - you may not be able to get Semglee in the country you are visiting
Your doctor, pharmacist or diabetes educator can provide you with some helpful information.
Tell your doctor if you are having trouble or difficulty with your eyesight.
Visit your doctor for regular checks of your eyes, feet, kidneys, heart, circulation, blood and blood pressure.
Carefully follow your doctor's and/ or dietician's advice on diet, drinking alcohol and exercise.
Things you must not do
Do not stop using Semglee unless your doctor tells you to.
Do not skip meals while using Semglee.
Do not use Semglee if you think it has been frozen or exposed to excessive heat (temperatures above 30°C).
Do not give Semglee to anyone else, even if they have the same condition as you.
Do not share needles or injection devices with other people, even if the needle has been changed. Do not reuse needles. You may give other people a serious infection, or get a serious infection from them.
Things to be careful of
Be careful driving or operating machinery until you know how Semglee affects you. Be careful not to let your blood sugar levels fall too low.
Tell your doctor if you drink alcohol. Alcohol may mask the symptoms of hypoglycaemia.
Tell your doctor if you are ill. Illness, especially with nausea and vomiting, may cause your insulin needs to change. Even if you are not eating, you still require insulin. You and your doctor should design an insulin plan for those times when you are sick.
If you become sick with a cold or flu, it is very important to continue using Semglee, even if you feel unable to eat your normal meal. If you have trouble eating solid foods, use sugar-sweetened drinks as a carbohydrate substitute or eat small amounts of bland food. Your diabetes educator or dietician can give you a list of foods to use for sick days.
Tell your doctor if you are exercising more than usual. Exercise may lower your need for Semglee. Exercise may also speed up the effect of a dose of Semglee, especially if the exercise involves the area of the injection site (e.g. the thighs should not be used for injection prior to jogging or running).
Tell your doctor if your diet changes. Changes in diet may cause your insulin needs to change.
Side effects
Tell your doctor, pharmacist or diabetes educator as soon as possible if you do not feel well while you are using Semglee.
Semglee helps most people with diabetes, but it may have unwanted side effects in a few people. All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical attention if you get some of the side effects.
Do not be alarmed by the following lists of side effects. You may not experience any of them.
Ask your doctor, pharmacist or diabetes educator to answer any questions you may have.
The most common side effect when using insulin is low blood sugar levels (hypoglycaemia - a "hypo").
Tell your doctor if you notice any of the following and they worry you:
- hypoglycaemia (mild to moderate)
- redness, swelling or itching at the injection site; usually these symptoms disappear within a few weeks during continued use
- a depression or thickening of the skin around the injection site (lipodystrophy); this can often occur if you inject too often at the same injection site
The above list includes the more common side effects of your medicine. They are usually mild and short-lived.
If any of the following happen, tell your doctor immediately or go to Accident and Emergency at your nearest hospital:
-
More severe symptoms of hypoglycaemia, including:
- disorientation
- seizures, fits or convulsions
- loss of consciousness -
Signs of a serious allergic reaction, including:
- skin rashes over a large part of the body
- shortness of breath, wheezing
- swelling of the face, lips or tongue
- fast pulse
- sweating
The above list includes some very serious side effects. You may need urgent medical attention or hospitalisation. These side effects are very rare.
Tell your doctor if you notice anything that is making you feel unwell.
Other side effects not listed above may also occur in some people.
After using Semglee
Storage
All medicines should be kept where children cannot reach them.
PRE-FILLED DISPOSABLE PENS
Before use, keep unopened Semglee pre-filled pens in a refrigerator where the temperature is between 2°C to 8°C. Do not allow it to freeze. Discard if frozen.
Before first use, store the pre-filled pen at room temperature for 1 to 2 hours.
Once in use, the opened, pre-filled pen should not be put in the refrigerator. It should be kept below 30°C away from direct heat and light.
Do not leave the needle attached to the pre-filled pen during storage
Always store the pre-filled pen with the cap on, to prevent contamination.
Discard the pre-filled pen within 28 days of first use. Pre-filled pens that are first carried as a spare for a while must also be discarded 28 days after being removed from the refrigerator.
Maintenance
Protect your pen from dust and dirt. You can clean the outside of your Semglee pen by wiping it with a damp cloth.
Do not soak, wash or lubricate the pen as this may damage it.
Handle your Semglee pen with care and avoid situations where it may be damaged. Dropping your pen can cause the cartridge to break or can damage the pen.
If you are concerned that your pen may be damaged, do not try to fix it. Use a new pen instead.
Disposal
Dispose of your insulin needles and disposable injection devices safely into a sharps container or as instructed by your healthcare professional. The used Semglee pen cannot be recycled.
If your doctor tells you to stop using Semglee or the expiry date has passed, ask your pharmacist what to do with any medicine that is left over.
Product description
What it looks like
Semglee is a pre-filled disposable pen containing a 3mL cartridge.
Each cartridge contains a clear, colourless solution.
Ingredients
Semglee contains the following active ingredient:
- insulin glargine (100 IU/mL)
It also contains the following inactive ingredients:
- meta-cresol
- glycerol
- zinc chloride
- hydrochloric acid
- sodium hydroxide
- water for injection
Supplier
Semglee is supplied in Australia by:
Alphapharm Pty Ltd (Mylan Australia)
(ABN 93 002 359 739)
Level 1, 30 The Bond
30-34 Hickson Road
Millers Point NSW 2000
Phone: (02) 9298 3999
www.mylan.com.au
Australian registration number:
Semglee pre-filled injection pen with 3 mL cartridge: AUST R 286207
This leaflet was prepared in March 2018.
Further information
You can get more information about diabetes and insulin from:
- Diabetes Australia:
freecall helpline 1300 136 588
www.diabetesaustralia.com.au
Semglee_cmi\Mar18/01
Published by MIMS November 2019



 Over the course of this 6 year study severe hypoglycaemia was reported in 5.7% of the insulin glargine group compared to 1.9% of the standard care group. The rates (per 100 patient-years) of confirmed all hypoglycaemia events, severe hypoglycaemia events and non-severe symptomatic hypoglycaemia are shown in Table 4.
Over the course of this 6 year study severe hypoglycaemia was reported in 5.7% of the insulin glargine group compared to 1.9% of the standard care group. The rates (per 100 patient-years) of confirmed all hypoglycaemia events, severe hypoglycaemia events and non-severe symptomatic hypoglycaemia are shown in Table 4. The median of the change in body weight from baseline to the last on-treatment visit was 2.2 kg greater in the insulin glargine group than in the standard care group i.e. weight gain of 1.4 kg in insulin glargine group compared to weight loss of 0.8 kg in standard care group.
The median of the change in body weight from baseline to the last on-treatment visit was 2.2 kg greater in the insulin glargine group than in the standard care group i.e. weight gain of 1.4 kg in insulin glargine group compared to weight loss of 0.8 kg in standard care group.
 The longer duration of Semglee is directly related to its slower rate of absorption and supports once daily subcutaneous administration. The time course of action of insulin and insulin analogues such as Semglee may vary considerably in different individuals or within the same individual but is, due to the lack of a peak, less variable with insulin glargine than with NPH insulin.
The longer duration of Semglee is directly related to its slower rate of absorption and supports once daily subcutaneous administration. The time course of action of insulin and insulin analogues such as Semglee may vary considerably in different individuals or within the same individual but is, due to the lack of a peak, less variable with insulin glargine than with NPH insulin. Semglee has not been studied in children below 2 years.
Semglee has not been studied in children below 2 years. In another Phase 3 study in patients with type 2 diabetes not using oral antidiabetic agents (Study 3006, n=518), a basal-bolus regimen of insulin glargine once daily at bedtime or NPH human insulin administered once or twice daily was evaluated for 28 weeks. Regular human insulin was used before meals as needed. Insulin glargine had similar effectiveness as either once or twice-daily NPH human insulin in reducing GHb and fasting glucose. Fewer patients treated with insulin glargine reported nocturnal hypoglycaemia from study month 2 to end of study (Table 8).
In another Phase 3 study in patients with type 2 diabetes not using oral antidiabetic agents (Study 3006, n=518), a basal-bolus regimen of insulin glargine once daily at bedtime or NPH human insulin administered once or twice daily was evaluated for 28 weeks. Regular human insulin was used before meals as needed. Insulin glargine had similar effectiveness as either once or twice-daily NPH human insulin in reducing GHb and fasting glucose. Fewer patients treated with insulin glargine reported nocturnal hypoglycaemia from study month 2 to end of study (Table 8).


 Median on-treatment HbA1c values ranged from 5.9 to 6.4% in the insulin glargine group, and 6.2% to 6.6% in the standard care group throughout the duration of follow-up. Median FPG at the end of study in the insulin glargine group was 5.4 mmol/L, and for the standard care group was 6.8 mmol/L.
Median on-treatment HbA1c values ranged from 5.9 to 6.4% in the insulin glargine group, and 6.2% to 6.6% in the standard care group throughout the duration of follow-up. Median FPG at the end of study in the insulin glargine group was 5.4 mmol/L, and for the standard care group was 6.8 mmol/L. The median of the change in body weight from baseline to the last on-treatment visit was 2.2 kg greater in the insulin glargine group than in the standard care group i.e. weight gain of 1.4 kg in insulin glargine group compared to weight loss of 0.8 kg in standard care group.
The median of the change in body weight from baseline to the last on-treatment visit was 2.2 kg greater in the insulin glargine group than in the standard care group i.e. weight gain of 1.4 kg in insulin glargine group compared to weight loss of 0.8 kg in standard care group.
 Molecular formula: C267H404N72O78S6. Molecular weight: 6063.
Molecular formula: C267H404N72O78S6. Molecular weight: 6063.