What is in this leaflet
This leaflet answers some common questions about SINGULAIR. It does not contain all the available information.
It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking SINGULAIR against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine. You may need to read it again.
What SINGULAIR is used for
SINGULAIR is used to prevent asthma symptoms, including those that occur during the day and at night-time. It also prevents the narrowing of airways triggered by exercise.
If you have seasonal allergic rhinitis (hay fever), SINGULAIR also treats your allergic rhinitis symptoms.
It can be used in children 2 years of age and older, teenagers and adults.
SINGULAIR tablets are not used to treat an acute attack of asthma. If an acute attack occurs, follow your doctor's instructions for your reliever medicine, and keep taking your SINGULAIR each night or as prescribed.
As a preventive medicine for asthma, SINGULAIR can be used alone or in combination with other preventive medicines, such as inhaled corticosteroids. Your doctor may reduce your dose of inhaled corticosteroid while you are taking SINGULAIR.
Asthma is a lung disease and has the following characteristics:
- narrowed airways causing breathing to become difficult
- inflamed airways, which means the lining of airways become swollen
- sensitive airways that react to many things, such as cigarette smoke, pollen, or cold air.
Symptoms of asthma include coughing, wheezing and chest tightness. Not all people with asthma wheeze. For some, coughing may be the only symptom of asthma. Symptoms often occur during the night or after exercise.
For further information about asthma, contact the Asthma Foundation in your state on 1800 645 130, or www.asthmaaustralia.org.au
Seasonal allergic rhinitis (also known as hay fever) is an allergic response often caused by airborne pollens from trees, grasses, and weeds. The daytime and night-time symptoms of seasonal allergic rhinitis typically may include: stuffy, runny, itchy nose; sneezing; watery, swollen, red, itchy eyes.
How SINGULAIR works
SINGULAIR belongs to a group of medicines called leukotriene receptor antagonists. It works by blocking substances in your lungs called leukotrienes that cause narrowing and swelling of airways. Blocking leukotrienes improves asthma symptoms and helps prevent asthma attacks. Leukotrienes also cause allergic rhinitis symptoms. By blocking leukotrienes, SINGULAIR improves seasonal allergic rhinitis symptoms.
Your doctor may have prescribed SINGULAIR for another reason. Ask your doctor if you have any questions about why SINGULAIR has been prescribed for you.
SINGULAIR is not addictive.
Before you take SINGULAIR
When you must not take it
Do not take SINGULAIR if:
- you have an allergy to SINGULAIR or any of the ingredients listed at the end of this leaflet
- the packaging is torn or shows signs of tampering
-
the expiry date on the pack has passed.
If you take this medicine after the expiry date has passed, it may not work.
If you are not sure whether you should start taking SINGULAIR, talk to your doctor.
Do not give SINGULAIR to children under 2 years of age. Safety and effectiveness in children younger than 2 years of age have not been studied.
In studies investigating the effect of SINGULAIR on the growth rate of children, it was shown that SINGULAIR did not affect the growth rate of children when given for up to 56 weeks in one study.
Before you start to take it
Tell your doctor if:
- you are pregnant or intend to become pregnant
SINGULAIR has not been studied in pregnant women.
- you are breast-feeding or plan to breast-feed
It is not known if SINGULAIR passes into breast milk.
- you have or have had any medical conditions
- your child has a condition called phenylketonuria
The 5 mg and 4 mg chewable tablets contain aspartame, corresponding to 0.842 mg phenylalanine in each 5 mg tablet and 0.674 mg in each 4 mg tablet.
- you have any allergies to any other medicines or any other substances, such as foods, preservatives or dyes.
If you have not told your doctor about any of the above, tell them before you take any SINGULAIR.
Taking other medicines
Some medicines may affect how SINGULAIR works, or SINGULAIR may affect how your other medicines work.
Tell your doctor if you are taking any other medicines, including medicines that you buy without a prescription from your pharmacy, supermarket or health food shop.
How to take SINGULAIR
How much to take
For patients with asthma and/or seasonal allergic rhinitis, take SINGULAIR only when prescribed by your doctor.
For adults and teenagers 15 years and older, the dose is one 10 mg film-coated tablet taken each day.
For children 6 to 14 years old, the dose is one 5 mg chewable tablet taken each day.
For children 2 to 5 years old, the dose is one 4 mg chewable tablet taken each day.
For patients with asthma, take SINGULAIR once a day in the evening.
For patients with seasonal allergic rhinitis, take SINGULAIR once a day as prescribed by your doctor.
Follow all directions given to you by your doctor carefully. They may differ from the information contained in this leaflet.
If you do not understand the instructions on the box, ask your doctor or pharmacist for help.
How to take it
SINGULAIR comes as three types of tablets:
- 10 mg film-coated tablets for adults and teenagers 15 years and older
- 5 mg chewable tablets for children 6-14 years old
- 4 mg chewable tablets for children 2-5 years old.
Swallow the 10 mg film-coated tablet with a glass of water.
Chew the 5 mg or 4 mg chewable tablets thoroughly and swallow. Do not swallow whole
When to take it
Asthma:
Take your SINGULAIR at bedtime each day. Taking your tablet at bedtime each day is expected to have the best effect. It will also help you remember when to take the tablets.
Seasonal allergic rhinitis:
Take your SINGULAIR once a day as prescribed by your doctor. Your doctor will advise you on the best time of the day to take your tablet.
Asthma and seasonal allergic rhinitis:
Take your SINGULAIR at bedtime each day if you have both asthma and seasonal allergic rhinitis.
It does not matter if you take SINGULAIR before or after food.
How long to take it
SINGULAIR helps control your asthma. Therefore SINGULAIR must be taken every day. Continue taking SINGULAIR for as long as your doctor prescribes.
If you forget to take it
Skip the dose you missed and take your next dose as usual.
Do not take a double dose to make up for the dose that you missed.
If you have trouble remembering to take your tablets, ask your pharmacist for some hints.
If you take too much (overdose)
Immediately telephone your doctor or Poisons Information Centre (telephone 13 11 26) for advice, if you think that you or anyone else may have taken too much SINGULAIR. Do this even if there are no signs of discomfort or poisoning.
The most common symptoms reported with overdose in adults and children include thirst, sleepiness, dilated pupils, hyperactivity, and stomach pain.
While you are using SINGULAIR
Things you must do
Continue taking SINGULAIR every day as directed by your doctor, even if you have no asthma symptoms or if you have an asthma attack.
If your asthma gets worse while taking SINGULAIR, tell your doctor immediately.
SINGULAIR is not for the treatment of acute asthma attacks. If an acute attack of asthma occurs, follow your doctor's instructions on what reliever medicine to use to relieve the attack.
All thoughts of suicide must be taken seriously. If you experience behaviour and mood-related changes including suicidal thoughts while taking SINGULAIR (see "Behavioural and mood related changes under Side Effects"), tell your doctor immediately.
In asthmatic patients treated with montelukast, very rare cases of a combination of allergic symptoms including a flu-like illness, pins and needles or numbness of arms or legs, and/or rash have been reported. Although it is unknown if montelukast can cause this condition, you must tell your doctor right away if you get one or more of these symptoms.
If you become pregnant while taking SINGULAIR, tell your doctor immediately.
If you are about to be started on any new medicine, tell your doctor and pharmacist that you are taking SINGULAIR.
Things you must not do
If you have been prescribed the 10 mg film-coated tablets, do not take two 5 mg chewable tablets in its place. If you have been prescribed the 5 mg tablets, do not take half a 10 mg tablet in its place.
The different strength tablets may not have the same effect, as they are absorbed slightly differently in the body.
Do not take SINGULAIR to relieve an acute asthma attack. In case of an acute asthma attack, follow your doctor's instructions on what reliever medicine to use.
Do not give SINGULAIR to anyone else, even if they have the same condition as you.
Side Effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking SINGULAIR.
SINGULAIR helps most people with asthma and/or seasonal allergic rhinitis, but it may have unwanted side effects in a few people. All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical treatment if you get some of the side effects.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor if you notice any of the following and they worry you:
- fluid retention
- nose bleed
- headache, dizziness, drowsiness
- feeling unusually weak or tired
- upper respiratory tract infection
- bedwetting in children
Muscle or nerve problems:
- muscle aches or cramps, joint pain
- decreased feeling or sensitivity, especially in the skin
- pins and needles/numbness
Stomach or bowel problems:
- stomach pain
- nausea, vomiting
- diarrhoea
Behaviour and mood-related changes, including suicidal thoughts and actions, have been reported in patients taking montelukast. If you or your child experience these changes while taking montelukast, tell your doctor.
Tell your doctor if you notice any of the following behaviour and mood-related changes:
- agitation, including aggressive behaviour or hostility
- anxiousness, depression (sad mood)
- disorientation, dream abnormalities, hallucinations (seeing or hearing things that are not there)
- insomnia, irritability, restlessness, sleep walking
- stuttering
- tremors
- disturbance in attention, memory impairment
- uncontrolled muscle movements
- obsessive-compulsive symptoms
These are usually mild side effects of SINGULAIR.
Tell your doctor immediately if you notice any of the following:
- suicidal thoughts and actions
- skin rash or itchiness
- increased tendency to bleed, bruising
- fast or irregular heart beats, also called palpitations
- swelling (inflammation) of the lungs
- symptoms of liver disease such as nausea, vomiting, loss of appetite, feeling generally unwell, fever, itching, yellowing of the skin and eyes, and dark coloured urine
These may be serious side effects. You may need urgent medical attention. Serious side effects are rare.
If any of the following happen, stop taking SINGULAIR and tell your doctor immediately or go to accident and emergency at your nearest hospital:
- swelling of the face, lips, mouth, throat or tongue which may cause difficulty in breathing or swallowing
- pinkish, itchy swellings on the skin, also called hives or nettlerash, severe skin reactions that may occur without warning
- seizure
These may be serious side effects. If you have them, you may be having a serious allergic reaction to SINGULAIR. You may need urgent medical attention. These side effects are rare.
Tell your doctor or pharmacist if you notice anything that is making you feel unwell.
Other side effects not listed above may also occur in some patients. Some of these side effects (for example, increased bleeding tendency, low blood platelet count) can only be detected when your doctor does tests from time to time to check your progress.
Tell your doctor if you notice any other effects.
After using SINGULAIR
Storage
Keep your tablets in the blister pack until it is time to take them. If you take the tablets out the blister pack they may not keep well.
Keep SINGULAIR in a cool dry place, away from light, where the temperature stays below 30°C.
Do not store it or any other medicine in the bathroom or near a sink.
Do not leave it in the car or on window sills. Heat and dampness can destroy some medicines.
Keep it where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking the tablets, or the tablets have passed their expiry date, ask your pharmacist what to do with any that are left over.
Product description
What it looks like
SINGULAIR comes as three types of tablets:
- 10 mg film-coated tablet - beige, rounded square tablet with SINGULAIR marked on one side and MSD 117 on the other.
- 5 mg chewable tablet - pink, round tablet with SINGULAIR marked on one side and MSD 275 on the other.
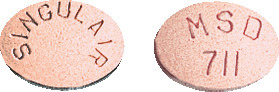
- 4 mg chewable tablet - pink, oval tablet with SINGULAIR marked on one side and MSD 711 on the other.
A box of SINGULAIR contains 14 or 28 tablets. To start treatment, SINGULAIR may also be supplied in packs of 4 tablets.
Ingredients
Active ingredient:
- 10 mg film-coated tablet contains 10 mg montelukast
- 5 mg chewable tablet contains 5 mg montelukast
- 4 mg chewable tablet contains 4 mg montelukast
Inactive ingredients:
- 10 mg film-coated tablets
- microcrystalline cellulose
- lactose monohydrate
- croscarmellose sodium
- hyprolose
- magnesium stearate
- hypromellose
- titanium dioxide
- iron oxide red
- iron oxide yellow
- carnauba wax
- 5 mg and 4 mg chewable tablets
- mannitol
- microcrystalline cellulose
- hyprolose
- croscarmellose sodium
- artificial cherry flavour aromolok 181612 (proprietary ingredient: 2916)
- magnesium stearate
- aspartame
- iron oxide red
SINGULAIR 10 mg film-coated tablets, and 5 mg and 4 mg chewable tablets do not contain gluten, sucrose, tartrazine or any other azo dyes. The 10 mg film-coated tablets contain lactose monohydrate; the 5 mg and 4 mg chewable tablets do not.
Supplier
SINGULAIR is supplied in Australia by:
Organon Pharma Pty Ltd
Building A 26 Talavera Road
MACQUARIE PARK NSW 2113
This leaflet was prepared in November 2022.
Australian Register Numbers:
10 mg - AUST R 61846
5 mg - AUST R 61847
4 mg - AUST R 74890
S-WPPI-MK-0476-MF--052019
RCN100002584
This CMI leaflet was current at the time of printing. To check if it has been updated, please view our website www.singulair.com.au or ask your pharmacist.
Published by MIMS December 2022

 Cumulatively, 544 patients were treated with Singulair for at least 6 months, 253 for one year and 21 for two years in clinical studies. With prolonged treatment, the adverse experience profile did not change.
Cumulatively, 544 patients were treated with Singulair for at least 6 months, 253 for one year and 21 for two years in clinical studies. With prolonged treatment, the adverse experience profile did not change.
 The efficacy of Singulair in the treatment of seasonal allergic rhinitis was investigated in a separate 4 week study in which the primary objective of the study was to determine the treatment effect of Singulair 10 mg administered once daily in the morning, compared to placebo, in patients with seasonal allergic rhinitis over the first 2 weeks of treatment. The efficacy of Singulair over the initial 2 weeks was significantly different from placebo and consistent with the effect observed in studies using evening dosing (see Table 4). Additionally, the effect over the entire 4 weeks was consistent with the 2 week results (see Figure 1).
The efficacy of Singulair in the treatment of seasonal allergic rhinitis was investigated in a separate 4 week study in which the primary objective of the study was to determine the treatment effect of Singulair 10 mg administered once daily in the morning, compared to placebo, in patients with seasonal allergic rhinitis over the first 2 weeks of treatment. The efficacy of Singulair over the initial 2 weeks was significantly different from placebo and consistent with the effect observed in studies using evening dosing (see Table 4). Additionally, the effect over the entire 4 weeks was consistent with the 2 week results (see Figure 1).
 The study was not designed for statistical comparison between Singulair and the active control (loratadine).
The study was not designed for statistical comparison between Singulair and the active control (loratadine).
