What is in this leaflet
This leaflet answers some common questions about SOOLANTRA.
It does not contain all the available information. It does not use the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you using SOOLANTRA against the benefits they expect it will have for you.
If you have any concerns about this medicine, ask your doctor or pharmacist.
Keep this leaflet with your medicine. You may need to read it again.
What SOOLANTRA is used for
SOOLANTRA contains the active substance ivermectin that belongs to a group of medicines called avermectins.
SOOLANTRA is used to treat pimples and spots found with rosacea.
Your doctor, however may prescribe it for another purpose.
Ask your doctor if you have any questions about why SOOLANTRA has been prescribed for you.
This medicine is available only with a doctor’s prescription.
SOOLANTRA is not addictive.
Before you use SOOLANTRA
When you must not use it
Do not use SOOLANTRA:
- if you have an allergy to any medicine containing ivermectin
- if you have an allergy to any of the ingredients listed at the end of this leaflet
- for any children under 18 years of age
Some of the symptoms of an allergic reaction may include:
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body
- rash, itching or hives of the skin
Do not use SOOLANTRA after the use by (expiry) date printed on the pack or if the packaging is torn or shows signs of tampering. If it has expired or is damaged, return it to your pharmacist for disposal.
Before you start to use it
Tell your doctor if you have allergies to any other medicines, foods, preservatives or dyes.
SOOLANTRA contains cetyl alcohol and stearyl alcohol which may cause local skin reactions (e.g. contact dermatitis), methyl hydroxybenzoate (E218) and propyl hydroxybenzoate (E217) which may cause allergic reactions (possibly delayed), and propylene glycol which may cause skin irritation.
Tell your doctor if you have or have had any of the following medical conditions:
- Kidney disease
- Liver disease
Tell your doctor if you are pregnant or plan to become pregnant or are breast-feeding. Your doctor can discuss with you the risks and benefits involved.
If you have not told your doctor about any of the above, tell him/her before you start taking SOOLANTRA.
SOOLANTRA should only be used on the skin of the face. At the start of the treatment, some patients may experience worsening of the symptoms of rosacea, however this is uncommon and usually resolves within 1 week of the treatment. Talk to your doctor if this happens.
SOOLANTRA should not be used on the following areas:
- eyes or eyelids
- mouth
- lips
If SOOLANTRA comes into contact with these areas, the area should be rinsed immediately with plenty of water.
SOOLANTRA is not recommended for use in children under 18 years.
Using other medicines
Tell your doctor or pharmacist if you are taking any other medicines, including any that you get without a prescription from your pharmacy, supermarket or health food shop. Your doctor and pharmacist have more information on medicines to be careful with or avoid while taking this medicine.
How to use SOOLANTRA
How much to use
Five small pea size amounts of SOOLANTRA is the daily recommended dose (approximately 1 gram).
Ask your doctor or pharmacist if you are not sure how much to apply. They will tell you exactly how much to use for each application.
Follow the instructions they give you.
How to use it
SOOLANTRA is only intended for use on the skin of the face.
It is recommended that SOOLANTRA be applied once per day after your usual cleansing routine, and before any cosmetics or sunscreens are applied.
Apply a small, pea size amount of SOOLANTRA to each of the following five areas of the face: forehead, chin, nose, and to each cheek, avoiding the eyes, eyelids, lips and mouth. The product should be applied smoothly and evenly as a thin layer across your face.
Hands should be washed after applying SOOLANTRA.
After applying SOOLANTRA use a non-comedogenic, broad spectrum, SPF 50+ sunscreen and wear protective clothing to protect your skin from UV rays.
Cosmetics can also be applied after SOOLANTRA has dried.
How to open the tube with a child-resistant cap.
To avoid spillage, do not squeeze the tube while opening or closing. Push down on the cap and turn in a counter clockwise (to the left) a quarter of a turn.
- Push down the cap
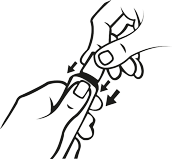
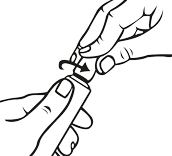
- twist in the direction of the arrow as shown below (counterclockwise).
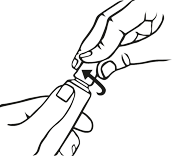
How to close the tube with a child-resistant cap.
Gently press down on the child resistant cap and twist to the right (clockwise).
How long to use it
Your doctor or pharmacist will tell you how long to use SOOLANTRA.
Continue using your medicine for as long as your doctor tells you. You should use SOOLANTRA daily over the treatment course. The doctor may decide that the treatment course should be repeated. The duration of treatment can vary from person to person and depends on the severity of your skin condition.
Pimples and spots will be reduced only after several applications of this medicine. It is important that you continue using SOOLANTRA as long as prescribed by your doctor.
If you are not sure how long to use SOOLANTRA, talk to your doctor or pharmacist.
If you forget to use it
If it is almost time for your next dose, skip the dose you missed and use your next dose when you are meant to.
Do not use a double dose to make up for the dose that you missed.
If you have trouble remembering to use your medicine, ask your pharmacist for some hints.
If you swallow it
Immediately telephone your doctor, or Poisons Information Centre (telephone 13 11 26), or go to Accident and Emergency at your nearest hospital, if you think you or anyone else may have taken too much SOOLANTRA.
Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
While you are using SOOLANTRA
Things you must do
Tell all doctors and pharmacists who are treating you that you are using SOOLANTRA.
If you feel that SOOLANTRA is not helping your condition, tell your doctor or pharmacist.
Tell your doctor if, for any reason, you have not used SOOLANTRA exactly as prescribed. Otherwise, your doctor may think that it was not effective and change your treatment unnecessarily.
If you become pregnant while using SOOLANTRA, tell your doctor.
Things you must not do
Do not use SOOLANTRA under dressings or on large areas of skin unless your doctor tells you.
Do not use SOOLANTRA in or near the eyes.
Do not give SOOLANTRA to anyone else, even if they have the same symptoms as yours.
Do not use SOOLANTRA to treat other complaints unless your doctor or pharmacist tells you to.
Things to be careful of
Avoid contact with the eyes, eyelids, lips and mouth. If SOOLANTRA comes into contact with these areas, the area should be rinsed immediately with plenty of water.
Ask your doctor or pharmacist if you are concerned about the length of time you have been using SOOLANTRA.
Side effects
All medicines may have some unwanted side effects. Sometimes they are serious, but most of the time they are not. Your doctor has weighed the risks of using this medicine against the benefits they expect it will have for you.
Do not be alarmed by this list of possible side effects. You may not experience any of them.
Tell your doctor if you notice any of the following and they worry you:
- skin burning sensation
- skin irritation
- itching of the skin
- dry skin
- redness
- Rosacea aggravation
- Swelling of the face
These are mild side effects of the medicine and are usually mild and short-lived.
If any of the following happen, stop using SOOLANTRA and tell your doctor immediately, or go to Accident and Emergency at your nearest hospital:
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body
- rash, itching or hives of the skin
- Liver enzyme elevations (ALAT/ASAT)
These are very serious side effects. If you have them, you may have had a serious allergic reaction to SOOLANTRA. You may need urgent medical attention or hospitalisation. Serious side effects are very rare.
Tell your doctor if you notice anything else that is making you feel unwell. Other side effects not listed above may occur in some patients.
Do not be alarmed by this list of possible side effects. You may not experience any of them.
After using SOOLANTRA
If you have queries about any aspect of your medicine, or any questions regarding the information in this leaflet, discuss them with your doctor or pharmacist.
Storage
Keep the medicine in a cool dry place where the temperature stays below 30°C. Do not freeze.
Do not store it, or any other medicine, in a bathroom, near a sink, or on a window-sill.
Do not leave it in the car. Heat and dampness can destroy some medicines.
Keep it where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop using SOOLANTRA or it has passed its expiry date, ask your pharmacist what to do with any that is left over.
Return any unused medicine to your pharmacist.
Product description
What it looks like
SOOLANTRA is a white to light yellow cream. It is supplied in plastic tube with a plastic cap containing 2 g, 15 g, 30 g, 45 g or 60 g of cream. For tube sizes greater than 2 g, the cap is a childresistant closure.
Not all pack sizes may be available.
Ingredients
Each gram of SOOLANTRA contains 10 mg (or 1.0% w/w) of ivermectin as the active ingredient.
Inactive ingredients:
- glycerol
- isopropyl palmitate
- carbomer copolymer (type B)
- dimethicone 20
- disodium edetate
- citric acid monohydrate
- cetyl alcohol
- stearyl alcohol
- ceteareth-20
- sorbitan stearate
- methyl hydroxybenzoate (E218)
- propyl hydroxybenzoate (E217)
- phenoxyethanol
- propylene glycol
- oleyl alcohol
- sodium hydroxide
- purified water.
Sponsor
Galderma Australia Pty Ltd
Suite 4, 13B Narabang Way
Belrose NSW 2085
Ph 1800 800 765
Distributed in New Zealand by:
Healthcare Logistics
58 Richard Pearse Drive
Airport Oaks
Auckland
Ph: 0800 174 104
Made in France
Australian Registration Number: AUST R 227125
® Registered Trademark
This leaflet was updated on 11 March 2020.
Published by MIMS May 2020
