SOVALDI®
| Consumer Medicine Information (CMI) summary |
The full CMI on the next page has more details. If you are worried about using this medicine, speak to your doctor or pharmacist.
| 1. Why am I using Sovaldi? |
Sovaldi contains the active ingredient sofosbuvir. SOVALDI is given with other medicines to treat hepatitis C virus (HCV) infection in adults 18 years and older.
For more information, see Section 1. Why am I using Sovaldi? in the full CMI.
| 2. What should I know before I use Sovaldi? |
Do not use if you have ever had an allergic reaction to Sovaldi or any of the ingredients listed at the end of the CMI.
Talk to your doctor if you have any other medical conditions, take any other medicines, or are pregnant or plan to become pregnant or are breastfeeding.
For more information, see Section 2. What should I know before I use Sovaldi? in the full CMI.
| 3. What if I am taking other medicines? |
Some medicines may interfere with Sovaldi and affect how it works.
A list of these medicines is in Section 3. What if I am taking other medicines? in the full CMI.
| 4. How do I use Sovaldi? |
The usual dose is one Sovaldi tablet orally, once daily. Sovaldi tablets can be taken with or without food.
More instructions can be found in Section 4. How do I use SOVALDI? in the full CMI.
| 5. What should I know while using Sovaldi? |
| Things you should do |
|
| Things you should not do |
|
| Driving or using machines |
|
| Looking after your medicine |
|
For more information, see Section 5. What should I know while using Sovaldi? in the full CMI.
| 6. Are there any side effects? |
Common side effects include headache, tiredness, diarrhoea and feeling sick (nausea).
For more information, including what to do if you have any side effects, see Section 6. Are there any side effects? in the full CMI.
SOVALDI®
Active ingredient: sofosbuvir
| Consumer Medicine Information (CMI) |
This leaflet provides important information about using Sovaldi. You should also speak to your doctor or pharmacist if you would like further information or if you have any concerns or questions about using Sovaldi.
Where to find information in this leaflet:
1. Why am I using Sovaldi?
2. What should I know before I use Sovaldi?
3. What if I am taking other medicines?
4. How do I use Sovaldi?
5. What should I know while using Sovaldi?
6. Are there any side effects?
7. Product details
| 1. Why am I using Sovaldi? |
Sovaldi contains the active ingredient sofosbuvir. Sovaldi works together with other medicines by lowering the amount of hepatitis C virus in your body and may lead to a cure of your HCV infection over at least 12 weeks.
Cure means the HCV virus is cleared from your blood (remains at an undetectable level) when measured 3 months after finishing all treatment.
Sovaldi does not protect against re-infection with the HCV virus if cure has been achieved.
Sovaldi is used to treat hepatitis C virus (HCV) infection in adults 18 years and older.
Hepatitis C is a virus that infects the liver.
| 2. What should I know before I use Sovaldi? |
Warnings
Do not use Sovaldi if:
- you are allergic to sofosbuvir, or any of the ingredients listed at the end of this leaflet.
- Always check the ingredients to make sure you can use this medicine.
Check with your doctor if you:
- Have liver problems, other than hepatitis C
- Have a current or previous infection with the hepatitis B virus since your doctor may want to monitor you more closely.
- Have HIV infection
- Have kidney problems or if you are on haemodialysis.
- Have diabetes
- Have any other medical condition.
During treatment, you may be at risk of developing certain side effects. It is important you understand these risks and how to monitor for them. See additional information under Section 6. Are there any side effects?
Pregnancy and breastfeeding
Check with your doctor if you are pregnant or intend to become pregnant. Sovaldi is commonly used together with ribavirin or with peginterferon alfa and ribavirin. Ribavirin can damage your unborn baby. It is therefore absolutely essential that you (and your partner) take all precautions not to get pregnant during this therapy. You and your partner must use an effective birth control method during ribavirin treatment and during the 6 months after ribavirin treatment. It is very important that you read the Pregnancy section in the ribavirin product information very carefully.
Talk to your doctor if you are breastfeeding or intend to breastfeed. It is not known whether sofosbuvir, the active ingredient of Sovaldi, pass into human breast milk.
Use in Children
Sovaldi is recommended for adults. Sovaldi has not been studied in children under 18 years of age.
| 3. What if I am taking other medicines? |
Tell your doctor or pharmacist if you are taking any other medicines, including any medicines, vitamins or supplements that you buy without a prescription from your pharmacy, supermarket or health food shop.
Tell your doctor if you take any of the following medicines:
- modafinil
- amiodarone used to treat heart conditions
- Warfarin or other similar medicines called vitamin K antagonists used to thin the blood.
- carbamazepine, phenytoin (medicines used to treat epilepsy and prevent seizures)
- rifampicin, rifapentine, rifabutin (antibiotics used to treat infections, including tuberculosis)
- St. John's Wort (Hypericum perforatum – herbal medicine used to treat depression)
- tipranavir used to treat HIV infection
SOVALDI may interact with these medicines. As a result, the amounts of SOVALDI or other medicines in your blood may be affected. This may stop your medicines from working properly, or make any side effects worse. In some cases your doctor may need to give you a different medicine or adjust the dose of medicine you are taking.
Check with your doctor or pharmacist if you are not sure about what medicines, vitamins or supplements you are taking and if these affect Sovaldi.
| 4. How do I use Sovaldi? |
How much to take
- The usual dose is one Sovaldi tablet orally, once daily.
- Sovaldi tablets can be taken with or without food.
- Follow the instructions provided and use Sovaldi until your doctor tells you to stop.
When to take Sovaldi
- Sovaldi should be taken at the same time each day will have the best effect. It will also help you remember when to take it.
If you forget to use Sovaldi
Sovaldi should be used regularly at the same time each day. If you miss your dose at the usual time, take your missed dose right away unless it is almost time for your next dose.
If it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to.
Do not take a double dose to make up for the dose you missed. Continue with your regular dosing schedule.
Do not stop taking Sovaldi unless your doctor tells you to. It is very important that you complete the full course of treatment to give the medicine the best chance to cure your hepatitis C virus infection.
If you use too much Sovaldi
If you think that you have used too much Sovaldi, you may need urgent medical attention.
You should immediately:
- phone the Poisons Information Centre
by calling 13 11 26 (Australia), or 0800 764 766 (New Zealand), or - contact your doctor, or
- go to the Emergency Department at your nearest hospital.
You should do this even if there are no signs of discomfort or poisoning.
| 5. What should I know while using Sovaldi? |
Things you should do
Remind any doctor, dentist or pharmacist you visit that you are using Sovaldi.
Tell your doctor as soon as possible if there is any worsening of your condition.
Things you should not do
- Do not give Sovaldi to anyone else, even if they have the same condition as you.
- Do not stop using this medicine without checking with your doctor
- Do not breastfeed.
Driving or using machines
Be careful before you drive or use any machines or tools until you know how Sovaldi affects you.
Looking after your medicine
- Keep your Sovaldi tablets in the bottle with the cap tightly closed until you take them. If you take SOVALDI tablets out of their pack they may not keep well.
- Keep Sovaldi tablets in a cool, dry place where it stays below 30°C.
Follow the instructions in the carton on how to take care of your medicine properly.
Store it in a cool dry place away from moisture, heat or sunlight.
Do not store it:
- in the bathroom or near a sink, or
- in the car or on window sills.
Keep it where young children cannot reach it.
Getting rid of any unwanted medicine
If you no longer need to use this medicine or it is out of date, take it to any pharmacy for safe disposal.
Do not use this medicine after the expiry date.
| 6. Are there any side effects? |
All medicines can have side effects. If you do experience any side effects, most of them are minor and temporary. However, some side effects may need medical attention.
See the information below and, if you need to, ask your doctor or pharmacist if you have any further questions about side effects.
Less serious side effects
| Less serious side effects | What to do |
| Speak to your doctor if you have any of these less serious side effects and they worry you. |
Serious side effects
| Serious side effects | What to do |
Signs of allergic reaction such as:
| Call your doctor straight away, or go straight to the Emergency Department at your nearest hospital if you notice any of these serious side effects. |
Tell your doctor or pharmacist if you notice anything else that may be making you feel unwell.
Other side effects not listed here may occur in some people.
Reporting side effects
After you have received medical advice for any side effects you experience, you can report side effects to the Therapeutic Goods Administration online at www.tga.gov.au/reporting-problems. By reporting side effects, you can help provide more information on the safety of this medicine.
Always make sure you speak to your doctor or pharmacist before you decide to stop taking any of your medicines.
| 7. Product details |
This medicine is only available with a doctor's prescription.
What Sovaldi contains
| Active ingredient (main ingredient) | sofosbuvir |
| Other ingredients (inactive ingredients) | mannitol, microcrystalline cellulose croscarmellose sodium silicon dioxide magnesium stearate. Film coating: polyvinyl alcohol titanium dioxide macrogoltalc purified iron oxide yellow. |
| Potential allergens | n/a |
Do not take this medicine if you are allergic to any of these ingredients.
What Sovaldi looks like
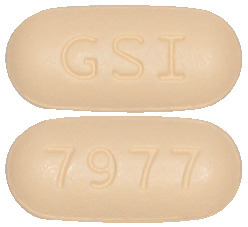
SOVALDI tablets are capsuled-shaped and yellow in colour. Each tablet has “GSI” on one side and “7977” on the other side of the tablet.
Sovaldi tablets are supplied in bottles containing 28 tablets.
AUST R 211019.
Who distributes Sovaldi
Australia
Gilead Sciences Pty Ltd
Level 6, 417 St Kilda Road Melbourne, Victoria 3004
New Zealand
c/- Grant Thornton New Zealand Limited, L4, 152 Fanshawe Street
Auckland 1010
This leaflet was prepared in February 2022.
Sovaldi, 7977 and GSI are trademarks of Gilead Sciences, Inc. or one of its related companies. Other brands listed are trademarks of their respective owners and are not trademarks of Gilead Sciences, Inc.
Published by MIMS April 2022