TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600
| Consumer Medicine Information (CMI) summary |
The full CMI on the next page has more details. If you are worried about using this medicine, speak to your doctor or pharmacist.
| 1. Why am I using TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600? |
TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600 contains the active ingredient tenofovir disoproxil maleate, emtricitabine and efavirenz. This medicine is used to treat Human Immunodeficiency Virus (HIV-1) infection in adults.
See Section 1. Why am I using TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600? in the full CMI.
| 2. What should I know before I use TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600? |
Do not use if you have ever had an allergic reaction to tenofovir disoproxil maleate, emtricitabine and efavirenz or any of the ingredients listed at the end of the CMI. Talk to your doctor if you have any other medical conditions, take any other medicines, or are pregnant or plan to become pregnant or are breastfeeding. For more information, see Section 2. What should I know before I use TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600? in the full CMI.
| 3. What if I am taking other medicines? |
Some medicines may interfere with this medicine and affect how it works. A list of these medicines is in Section 3. What if I am taking other medicines? in the full CMI.
| 4. How do I use TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600? |
- The usual dose of TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600 is one tablet once a day.
Details are in Section 4. How do I use TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600? in the fullCMI.
| 5. What should I know while using TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600? |
| Things you should do |
|
| Things you should not do |
|
| Driving or using machines |
|
| Drinking alcohol |
|
| Looking after your medicine |
|
For more information, see Section 5. What should I know while using TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600? in the full CMI.
| 6. Are there any side effects? |
The common side effects are dizziness, headache, trouble sleeping, drowsiness, trouble concentrating, unusual dreams, rash, tiredness, upset stomach, vomiting, gas and diarrhoea. Tell your doctor right away if any of these side effects bother you.
For more information, including what to do if you have any side effects, see Section 6. Are there any side effects? in the full CMI.
TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600
Active ingredients: tenofovir disoproxil maleate, emtricitabine, efavirenz
| Consumer Medicine Information (CMI) |
This leaflet provides important information about using TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600. You should also speak to your doctor or pharmacist if you would like further information or if you have any concerns or questions about using TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600.
Where to find information in this leaflet:
1. Why am I using TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600?
2. What should I know before I use TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600?
3. What if I am taking other medicines?
4. How do I use TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600?
5. What should I know while using TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600?
6. Are there any side effects?
7. Product details
| 1. Why am I using TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600? |
TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600 contains the active ingredients tenofovir disoproxil maleate, emtricitabine and efavirenz. These are combined in one tablet to help control Human Immunodeficiency Virus (HIV-1) infection.
Tenofovir disoproxil and emtricitabine belong to a group of antiviral medicines known as nucleoside and nucleotide reverse transcriptase inhibitors (NRTI). Efavirenz belongs to a group of antiviral medicines known as non-nucleoside reverse transcriptase inhibitors (NNRTI).
TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600 is used to treat HIV-1 infection in adults. This medicine can be taken alone or in combination with other anti-HIV medicines.
Do not take TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600 if you are under the age of 18 years.
Do not take TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600 if you are over the age of 65 before discussing with your doctor.
How TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600 works
HIV-1 infection destroys CD4 T cells, which are important to the immune system. The immune system helps fight infection. After a large number of T cells are destroyed, acquired immune deficiency syndrome (AIDS) may develop.
TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600 helps block HIV-1 reverse transcriptase, a viral chemical in your body (enzyme) that is needed for HIV-1 to multiply. TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600 lowers the amount of HIV-1 in the blood (viral load). TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600 may also help to increase the number of T cells (CD4+ cells), allowing your immune system to improve. Lowering the amount of HIV-1 in the blood lowers the chance of death or infections that happen when your immune system is weak (opportunistic infections).
TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600 does not cure HIV-1 infection or AIDS.
The long-term effects of TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600 are not known at this time. People taking TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600 may still get opportunistic infections or other conditions that happen with HIV-1 infection.
Opportunistic infections are infections that develop because the immune system is weak.
Some of these conditions are:
- pneumonia,
- herpes virus infections, and
- Mycobacterium avium complex (MAC) infections.
This medicine is only available from a pharmacist after it has been prescribed by a doctor who specialises in the treatment of HIV-1 infection.
If you wish to continue receiving treatment with TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600, it is important you remain under the care of a hospital or doctor who specialises in the treatment of HIV-1 infection.
TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600 has not been shown to lower your chance of passing HIV to others through sexual contact, sharing needles, or being exposed to your blood.
Do not share needles or other injection equipment.
Do not share personal items that can have blood or body fluids on them, like toothbrushes or razor blades.
Do not have any kind of sex without protection.
| 2. What should I know before I use TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600? |
Warnings
Do not use TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600 if:
- you are allergic to tenofovir disoproxil maleate, tenofovir disoproxil fumarate, emtricitabine, efavirenz or any of the ingredients listed at the end of this leaflet.
- Some of the symptoms of an allergic reaction may include shortness of breath, wheezing or difficulty breathing, rash, itching or hives on the skin, swelling of the face, lips, tongue or other parts of the body.
- Always check the ingredients to make sure you can use this medicine.
- Do not use TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600 for a condition for which it was not prescribed.
- Do not share TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600 with other people even if they have the same symptoms that you have. It may harm them.
Check with your doctor if you have:
- kidney problems or are undergoing kidney dialysis treatment
- bone problems
- liver problems, including Hepatitis B Virus (HBV) infection
- HIV infection. Your doctor may want to do tests to check your liver while you take TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600
- ever had mental illness or are using recreational drugs or alcohol
- ever had seizures or are taking medicine for seizures
- ever had a serious allergic drug reaction involving the skin (e.g. Stevens Johnson syndrome)
- any medical conditions.
During treatment, you may be at risk of developing certain side effects. It is important you understand these risks and how to monitor for them. See additional information under Section 6. Are there any side effects?
Pregnancy and breastfeeding
Check with your doctor if you are pregnant or intend to become pregnant.
Women taking TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600 should not become pregnant. Serious birth defects have been seen in the babies of animals and women treated with efavirenz (a component of TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600) during pregnancy. It is not known whether efavirenz caused these defects.
Women of child-bearing age receiving treatment with TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600 should not rely only on hormone-based birth control, such as pills, injections or implants.
TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600 may make these contraceptives ineffective. Women must use a reliable form or barrier contraception (such as a condom or diaphragm), even if they also use other methods of birth control.
Talk to your doctor if you are breastfeeding or intend to breastfeed.
Do not breast-feed if you are taking TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600.
It is recommended that nursing mothers do not breast-feed during treatment with TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600. In general, women infected with HIV should not breast-feed their infants in order to avoid transmission of HIV to their newborn infant.
The active substances in this medicine (tenofovir and emtricitabine and efavirenz) have been found in breast milk at low concentrations.
Do not breast-feed if you have HIV or HBV.
If you are a woman who has or will have a baby, talk with your doctor or pharmacist about the best way to feed your baby. If your baby does not already have HIV or HBV, there is a chance that the baby can get HIV or HBV through breast-feeding. In general, women infected with HIV should not breast-feed their infants in order to avoid transmission of HIV to their newborn infant.
| 3. What if I am taking other medicines? |
Tell your doctor or pharmacist if you are taking any other medicines, including any medicines, vitamins or supplements that you buy without a prescription from your pharmacy, supermarket or health food shop.
TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600 may change the effect of other medicines, including the ones for HIV-1 and may cause serious side effects.
Your doctor may change your other medicines or change their doses. Other medicines, including herbal products may affect TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600.
For this reason, it is very important to let your doctor or pharmacist know what medications, herbal supplements, or vitamins you are taking.
Do not take any other medicines, including prescription or non-prescription medicines and herbal products, without checking with your doctor.
The following medicines may cause serious or life-threatening side effects when taken with TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600.
Do not take elbasvir/grazoprevir with TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600.
It may lose its effect and reduce your response to elbasvir/grazoprevir.
Do not take St. Johns wort (Hypericum perforatum) or products containing St. Johns wort with TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600.
St. John's wort is a herbal product sold as a dietary supplement. Talk to your doctor or pharmacist if you are taking or are planning to take St. John's wort. Taking St. John's wort may decrease TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600 levels and lead to increased viral load and possible resistance to TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600 or cross-resistance to other anti-HIV drugs.
Voriconazole should not be taken with TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600.
It may lose its effect or may increase the chance of having side effects from TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600.
Do not take TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600 if you are already taking any other medicines that contain the same active ingredients.
Do not take TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600 if you are taking other medicines that contain:
- Efavirenz
- Lamivudine
Do not take TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600 to treat your HIV infection if you are also taking adefovir dipivoxil.
It is also important to tell your doctor if you are taking any of the following:
- Bepridil, cisapride (e.g. Prepulsid), midazolam (e.g. Hypnovel), pimozide (e.g. Orap), triazolam (e.g. Halcion), ergot medications (e.g. Cafergot).
- Saquinavir (e.g. Invirase), clarithromycin (e.g. Klacid); these medicines may need to be replaced with another medicine when taken with TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600.
- Calcium channel blockers such as diltiazem (e.g. Vasocardol) and others; indinavir (e.g. Crixivan); methadone, rifabutin (e.g. Mycobutin), rifampin (e.g. Rifadin).
- Lipid-lowering medicines such as atorvastatin (e.g. Lipitor), pravastatin (e.g. Pravachol) or simvastatin (e.g. Zocor).
- Antidepressant medicines such as sertraline (e.g. Zoloft) or bupropion (e.g. Zyban); immunosuppressant medicines cyclosporine (e.g. Neoral), tacrolimus (e.g. Prograf) or sirolimus (e.g. Rapamune); these medicines may need to have their dose changed when taken with TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600.
- Didanosine (also known as ddI or Videx); tenofovir disoproxil (a component of TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600) may increase the amount of didanosine in your blood, which could result in more side effects. You may need to be monitored more carefully if you are taking didanosine and TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600 together. Also, the dose of didanosine may need to be changed.
- Atazanavir sulphate (e.g. Reyataz) or lopinavir/ritonavir (e.g. Kaletra); these medicines may increase the amount of tenofovir disoproxil (a component of TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600) in your blood which could result in more side effects. Atazanavir sulphate is not recommended with TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600. You may need to be monitored more carefully if you are taking TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600 and lopinavir/ritonavir together. Also, the dose of lopinavir/ritonavir may need to be changed.
- Other HIV medications including amprenavir (e.g. Agenerase), ritonavir (e.g. Norvir), fosamprenavir calcium (e.g. Telzir), raltegravir (e.g. Isentress), ritonavir or maraviroc (e.g. Celsentri), Also, the dose of maraviroc may need to be changed.
- Medicine for seizures such as phenytoin (e.g. Dilantin), carbamazepine (e.g. Tegretol) or phenobarbital (phenobarbitone); your doctor may want to switch you to another medicine or check drug levels in your blood from time to time.
- Antifungal medications such as itraconazole (e.g. Sporanox) and posaconazole (e.g. Noxafil); these medicines may need to be replaced with another medicine when taken with TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600.
- Hepatitis C antiviral agents such as ledipasvir/sofosbuvir (e.g. HARVONI), or sofosbuvir/velpatasvir (e.g. EPCLUSA), sofosbuvir/velpatasvir/voxilapreir (e.g. VOSEVI), simeprevir (e.g Olysio), bocepravir (e.g. Victrelis) and telaprevir (e.g. Incivek).
- Antimalarial Agents such as artemether/lumefantrine (Coartem/Riamet).
These are not all the medicines that may cause problems if you take TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600. Be sure to tell your healthcare provider about all medicines that you take.
It is a good idea to keep a complete list of all the medicines that you take.
Make a new list when medicines are added or stopped. Give copies of this list to your doctor or pharmacist every time you visit your doctor or fill a prescription.
| 4. How do I use TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600? |
How much to take
- The usual dose of TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600 is one tablet once a day.
- Take the exact amount of TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600 your doctor has prescribed for you.
- Never change the dose on your own.
- Do not stop this medicine unless your healthcare provider tells you to stop.
- Stay under a doctor's care when taking TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600.
- Do not change your treatment or stop treatment without first talking with your doctor.
When to take TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600
- Taking TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600 at bedtime may make some side effects less bothersome.
How to take TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600
- Always take TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600 on an empty stomach.
- Swallow TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600 with water.
If you forget to use TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600
It is important that you do not miss any doses of TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600.
If you miss a dose of TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600, take the missed dose as soon as possible and then take your next scheduled dose at its regular time.
If it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to.
Do not take a double dose to make up for the dose you missed.
If you think that you have used too much TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600, you may need urgent medical attention.
You should immediately:
- phone the Poisons Information Centre (Australia telephone 13 11 26); New Zealand telephone 0800 POISON or 0800 764 766 for advice, or
- contact your doctor, or
- go to the Emergency Department at your nearest hospital.
You should do this even if there are no signs of discomfort or poisoning.
| 5. What should I know while using TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600? |
Things you should do
Tell your doctor or pharmacist that you are taking TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600 if you are about to be started on any other medicines.
Your doses may need adjustment.
When your TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600 supply starts to run low, get more from your doctor or pharmacy.
This is very important because the amount of virus in your blood may increase if the medicine is stopped for even a short time. The virus may develop resistance to TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600 and become harder to treat.
Things you should not do
Avoid doing things that can spread HIV infection since TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600 does not stop you from passing the HIV infection to others.
Do not take TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600 if the packaging is torn or shows signs of tampering.
Driving or using machines
Be careful before you drive or use any machines or tools until you know how TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600 affects you.
If you are dizzy, have trouble concentrating, or are drowsy, avoid activities that may be dangerous, such as driving or operating machinery.
Drinking alcohol
Tell your doctor if you drink alcohol.
Taking TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600 with alcohol or other medicines causing similar side effects as TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600, such as drowsiness, may increase those side effects.
Looking after your medicine
- Keep your TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600 tablets in the bottle with the cap tightly closed until you take them.
Follow the instructions on the bottle on how to take care of your medicine properly.
Store it in a cool dry place below 25°C away from moisture, heat or sunlight; for example, do not store it:
- in the bathroom or near a sink, or
- in the car or on window sills.
Keep it where young children cannot reach it.
A locked cupboard at least one-and-a half metres above the ground is a good place to store them.
Getting rid of any unwanted medicine
If you no longer need to use this medicine or it is out of date, take it to any pharmacy for safe disposal.
Do not use this medicine after the expiry date.
| 6. Are there any side effects? |
All medicines can have side effects. If you do experience any side effects, most of them are minor and temporary. However, some side effects may need medical attention.
See the information below and, if you need to, ask your doctor or pharmacist if you have any further questions about side effects.
Common side effects
| Common side effects | What to do |
These side effects may be reduced if you take TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600 at bedtime on an empty stomach. They also tend to go away after you have taken the medicine for a few weeks. If you have these common side effects, such as dizziness, it does not mean that you will also have serious psychiatric problems, such as severe depression, strange thoughts or angry behaviour. It is possible that these symptoms may be more severe if TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600 is used with alcohol or mood altering (recreational) drugs.
Other common side effects:
| Speak to your doctor if you have any of these common side effects and they worry you. |
Other possible side effects:
Changes in body fat develop in some people receiving anti HIV-1 therapy. These changes may include an increased amount of fat in the upper back and neck ('buffalo hump'), in the breasts and around the trunk. Loss of fat from the legs, arms and face may also happen. The cause and long-term health effects of these fat changes are not known.
Sleeping problems (including difficulty to fall asleep or sleepiness).
Skin discolouration (small spots or freckles) may also occur with TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600.
Serious side effects
| Serious side effects | What to do |
Lactic Acidosis (build-up of an acid in the blood):
(hepatotoxicity):
These side effects may be due to a condition called hepatotoxicity with liver enlargement (hepatomegaly) and fat deposits in the liver (steatosis) which sometimes occurs in patients taking anti HIV-1 medicines. You may be more likely to get lactic acidosis or liver problems if you are female, very overweight (obese), or have been taking similar nucleoside analog-containing medicines, like TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600, for a long time.Pancreatitis:
| Call your doctor straight away, or go straight to the Emergency Department at your nearest hospital if you notice any of these serious side effects. |
Hepatic Flares
If you have HIV-1 infection and hepatitis B virus (HBV) infection you should not stop your TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600 treatment without first discussing this with your doctor, as some patients have had blood tests or symptoms indicating a worsening of their hepatitis ("hepatic flare") after stopping individual components (tenofovir disoproxil, and emtricitabine) of TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600.
You may require medical exams and blood tests for several months after stopping treatment. TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600 is not approved for the treatment of HBV, so you must discuss your HBV therapy with your doctor.
Serious Psychiatric Problems
A small number of patients may experience severe depression, strange thoughts, or angry behaviour while taking TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600.
Some patients have thoughts of suicide and a few have actually committed suicide.
These problems may occur more often in patients who have had mental illness.
Contact your doctor right away if you think you are having these psychiatric problems, so your doctor can decide if you should continue to take TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600.
Kidney Problems
If you have had kidney problems in the past or take other medicines that can cause kidney problems, your doctor should do regular blood tests to check your kidneys.
Changes in Bone Mineral Density (thinning bones)
It is not known whether long-term use of TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600 will cause damage to your bones. If you have had bone problems in the past, your doctor may need to do tests to check your bone mineral density or may prescribe medicines to help your bone mineral density.
Allergy
Some people are allergic to medicines.
If you have any of the following symptoms soon after taking your medicine, DO NOT TAKE ANY MORE Tenofovir TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600 and tell your doctor IMMEDIATELY or go to the accident and emergency department at your nearest hospital.
- Skin troubles such as lumpy skin rash or "hives"
- Swelling of the face, lips, mouth or throat which may cause difficulty in swallowing or breathing
- Wheezing, chest pain or tightness
- Fainting
These are very serious side effects. If you have them, you may have a serious allergic reaction.
You may need urgent medical attention or hospitalisation. Hypersensitivity reactions are very rare.
Contact your doctor before stopping TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600 because of side effects or for any other reason.
Tell your doctor or pharmacist if you notice anything else that may be making you feel unwell.
Other side effects not listed here may occur in some people.
Reporting side effects
After you have received medical advice for any side effects you experience, you can report side effects to the Therapeutic Goods Administration online at www.tga.gov.au/reporting-problems or New Zealand at nzphvc.otago.ac.nz/reporting. By reporting side effects, you can help provide more information on the safety of this medicine.
Always make sure you speak to your doctor or pharmacist before you decide to stop taking any of your medicines.
| 7. Product details |
This medicine is only available with a doctor's prescription.
What TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600 contains
| Active ingredient (main ingredient) | tenofovir disoproxil maleate emtricitabine efavirenz |
| Other ingredients (inactive ingredients) | colloidal anhydrous silica croscarmellose sodium ferric oxide hyprolose lactose monohydrate magnesium stearate microcrystalline cellulose OPADRY II complete film coating system 85F540043 PINK sodium metabisulfite |
| Potential allergens | sugars as lactose traces of sulfites |
Do not take this medicine if you are allergic to any of these ingredients.
What TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600 looks like
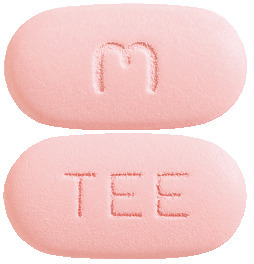
TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600 is capsule shaped and pink in colour. Each tablet is debossed with "M" on one side of the tablet and "TEE" on the other side. (AUST R 280721).
Who distributes TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600
Alphapharm Pty Ltd trading as Viatris
Level 1, 30 The Bond
30-34 Hickson Road
Millers Point NSW 2000
www.viatris.com.au
Phone: 1800 274 276
This leaflet was prepared in April 2022.
TENOFOVIR DISOPROXIL/EMTRICITABINE/EFAVIRENZ VIATRIS 300/200/600_cmi\Apr22/00
Published by MIMS June 2022