What is in this leaflet
Read this leaflet carefully before you start taking Tenofovir GH. Also, read it each time you get your Tenofovir GH prescription refilled, in case something has changed.
This information does not take the place of talking with your doctor or pharmacist when you start this medicine and at check-ups. You should stay under a doctor’s care when taking Tenofovir GH.
It is extremely important that you do not change or stop your medicine without first talking with your doctor or pharmacist (see 'How should I take Tenofovir GH and 'Side effects').
Talk to your doctor or pharmacist if you have any questions about Tenofovir GH.
What Tenofovir GH is used for
Tenofovir GH is an antiviral medication used to treat two different viruses: Chronic Hepatitis B (CHB) and Human Immunodeficiency Virus (HIV) infection.
Tenofovir GH is a type of medicine called an HBV polymerase inhibitor and a nucleotide analogue reverse transcriptase inhibitor (NRTI).
How Tenofovir GH works in the treatment of CHB
Tenofovir GH is used to treat CHB (an infection with hepatitis B virus [HBV]) in adults and paediatric patients aged 12 years and older and weighing at least 35 kg.
Tenofovir GH works by interfering with the normal working of enzymes (HBV DNA polymerase) that are essential for HBV to reproduce itself. Tenofovir GH may help lower the amount of hepatitis B virus in your body by lowering the ability of the virus to multiply and infect new liver cells and can improve the inflammation and scar tissue caused by the hepatitis B virus in your liver. Lowering the amount of virus in your body may reduce the chance of developing cirrhosis, liver failure and liver cancer.
We do not know how long Tenofovir GH may help treat your hepatitis.
Sometimes viruses change in your body and medicines no longer work. This is called drug resistance.
How Tenofovir GH works in the treatment of HIV-Infection
Tenofovir GH is also used to treat HIV infection in adults and paediatric patients aged 12 years and older and weighing at least 35 kg. Tenofovir GH is always used in combination with other anti-HIV medicines to treat people with HIV-1 infection.
HIV infection destroys CD4 (T) cells, which are important to the immune system. After a large number of T cells are destroyed, acquired immune deficiency syndrome (AIDS) develops. Tenofovir GH helps to block HIV-1 reverse transcriptase, a chemical (enzyme) in your body that is needed for HIV-1 to multiply.
Tenofovir GH lowers the amount of HIV 1 in the blood (called viral load) and may help to increase the number of T cells (called CD4 cells). Lowering the amount of HIV-1 in the blood lowers the chance of death or infections that happen when your immune system is weak (opportunistic infections).
You do not have to have HIV-infection to be treated with Tenofovir GH for HBV and vice versa.
Use in Children
Tenofovir GH is for adults and paediatric patients aged 12 years and older and weighing at least 35 kg.
Does Tenofovir GH cure HIV or AIDS
Tenofovir GH does not cure HIV infection or AIDS.
The long-term effects of Tenofovir GH are not known at this time. People taking Tenofovir GH may still get opportunistic infections or other conditions that happen with HIV-1 infection.
Opportunistic infections are infections that develop because the immune system is weak. Some of these conditions are:
- pneumonia;
- herpes virus infections; and
- Mycobacterium avium complex (MAC) infection.
Does Tenofovir GH reduce the risk of passing HIV-1 or HBV to others
Tenofovir GH does not reduce the risk of passing HIV-1 or HBV to others through sexual contact or blood contamination.
Continue to practice safe sex and do not use or share dirty needles.
Before you take Tenofovir GH
When you must not take it
Together with your doctor, you need to decide whether Tenofovir GH is right for you.
Do not take Tenofovir GH if you are allergic to any of the ingredients listed at the end of this leaflet under the heading “Product Description”.
Do not take Tenofovir GH if you are already taking any other medicines that contain the same active ingredients.
Do not take Tenofovir GH if you are already taking adefovir dipivoxil to treat hepatitis B virus (HBV) infection.
Do not take Tenofovir GH if you are already taking tenofovir alafenamide to treat HIV or hepatitis B virus (HBV) infection.
Before you start to take it
Tell your doctor if you are pregnant or planning to become pregnant.
The effects of Tenofovir GH on pregnant women or their unborn babies are not known.
Tell your doctor if you are breast-feeding.
Do not breast-feed if you are taking Tenofovir GH. The active substance in this medicine (tenofovir disoproxil) has been found in breast milk at low concentrations.
Do not breast-feed if you have HIV or HBV.
If you are a woman who has or will have a baby, talk with your doctor or pharmacist about the best way to feed your baby.
If your baby does not already have HIV or HBV, there is a chance that the baby can get HIV or HBV through breast-feeding.
Tell your doctor if you have kidney problems.
Tell your doctor if you have bone problems.
Tell your doctor if you have liver problems, including HBV and hepatitis C virus (HCV).
Tell your doctor if you have HIV infection.
Tell your doctor or pharmacist about all your medical conditions.
Taking other medicines
Tell your doctor or pharmacist about all the medicines you take, including prescription and non-prescription medicines and dietary supplements.
Especially tell your doctor or pharmacist if you take:
- VOSEVI (sofosbuvir/velpatasvir/ voxilaprevir), HARVONI (ledipasvir/sofosbuvir) or EPCLUSA (sofosbuvir/ velpatasvir) to treat your HCV infection. These medicines may increase the amount of VIREAD in your blood, which could result in additional or more intense side effects (see ‘Side Effects’).
- VIDEX, VIDEX EC (didanosine). Tenofovir GH may increase the amount of VIDEX in your blood. You may need to be followed more carefully if you are taking VIDEX and Tenofovir GH together. If you are taking VIDEX and Tenofovir GH together, your doctor may need to reduce your dose of VIDEX.
- REYATAZ (atazanavir sulfate) or KALETRA (lopinavir/ritonavir). These medicines may increase the amount of Tenofovir GH in your blood, which could result in more side effects (see ‘Side Effects’). You may need to be followed more carefully if you are taking Tenofovir GH and REYATAZ or KALETRA together. Tenofovir GH may decrease the amount of REYATAZ in your blood. If you are taking Tenofovir GH and REYATAZ together you should also be taking NORVIR (ritonavir).
It is a good idea to keep a complete list of all the medicines that you take.
Make a new list when medicines are added or stopped. Give copies of this list to your doctor or pharmacist every time you visit your doctor or fill a prescription.
How to take Tenofovir GH
Stay under a doctor’s care when taking Tenofovir GH.
Do not change your treatment or stop treatment without first talking with your doctor.
Take Tenofovir GH exactly as your doctor prescribed it.
Follow the directions from your doctor or pharmacist, exactly as written on the label.
Set up a dosing schedule and follow it carefully.
How much to take
The usual dose of Tenofovir GH is one tablet once a day.
If you have kidney problems, your doctor may recommend that you take Tenofovir GH less frequently.
How to take it
Tenofovir GH is best taken with a meal or just afterwards, however taking it without food should not reduce the effectiveness of the medicine.
If you are taking Tenofovir GH to treat HIV or if you have HIV and HBV co-infection and are taking Tenofovir GH, always take Tenofovir GH in combination with other anti-HIV medicines.
Tenofovir GH and other medicines like Tenofovir GH, may be less likely to work in the future if you are not taking Tenofovir GH with other anti-HIV medicines because you may develop resistance to those medicines. If you have any questions about what medicines you should or should not be taking, please see your doctor or pharmacist.
If you have been given Tenofovir GH to treat CHB, you are advised to get a HIV test before you start taking Tenofovir GH and at any time after that when there is a chance you were exposed to HIV.
When your Tenofovir GH supply starts to run low, get more from your doctor or pharmacist.
This is very important because the amount of virus in your blood may increase if the medicine is stopped for even a short time. The virus may develop resistance to Tenofovir GH, and may become harder to treat. If you are taking Tenofovir GH to treat CHB, stopping treatment may result in very severe hepatitis and serious liver problems (see 'Side effects').
Only take medicine that has been prescribed specifically for you.
Do not give Tenofovir GH to others or take medicine prescribed for someone else.
If you forget to take it
It is important that you do not miss any doses.
If you miss a dose of Tenofovir GH, take it as soon as possible and then take your next scheduled dose at its regular time.
If it is almost time for your next dose, do not take the missed dose. Wait and take the next dose at the regular time. Do not double the next dose.
If you take too much (overdose)
Immediately telephone your doctor or Poisons Information Centre (telephone 13 11 26), or in New Zealand the Poisons Centre (telephone 0800 764 766) or go to the accident and emergency department at your nearest hospital if you think you or anyone else may have taken too many Tenofovir GH tablets. Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
While you are taking Tenofovir GH
Things you must not do
Do not breastfeed (see 'Before you start to take it').
Things to be careful of
Some patients taking Tenofovir GH have experienced dizziness. Make sure you know how you react to Tenofovir GH before you drive a car, operate machinery or do anything else that could be dangerous if you are dizzy.
Side effects
In clinical studies in patients with HIV, the most common side effects of Tenofovir GH are:
- diarrhoea;
- nausea;
- vomiting;
- dizziness.
Less common side effects of Tenofovir GH are:
- flatulence (intestinal gas).
In clinical studies in patients with CHB the only common side effect of Tenofovir GH is:
- nausea.
Marketing experience has shown other side effects reported since Tenofovir GH has been marketed include:
- low blood phosphate;
- shortness of breath;
- increased liver enzymes;
- increased amylase;
- inflammation of the liver;
- stomach pain;
- inflammation of the pancreas;
- rash;
- weakness.
Tenofovir GH may cause the following other side effects:
Kidney Problems
Some patients treated with Tenofovir GH have had kidney problems. If you have had kidney problems in the past or need to take another drug that can cause kidney problems, your doctor may need to perform additional blood tests. Kidney problems may be associated with muscle problems and softening of the bones.
Changes in Bone Mineral Density
Laboratory tests show changes in the bones of patients treated with Tenofovir GH. It is not known whether long-term use of Tenofovir GH will cause damage to your bones. If you have had bone problems in the past, your doctor may need to perform additional tests or may suggest additional medication.
Lactic Acidosis
Some patients taking antiviral drugs like Tenofovir GH have developed a condition called lactic acidosis (a build-up in the blood of lactic acid, the same substance that causes your muscles to burn during heavy exercise). Symptoms of lactic acidosis include nausea, vomiting, unusual or unexpected stomach discomfort, and weakness.
If you notice these symptoms or if your medical condition changes suddenly, call your doctor right away.
Hepatic Flares
It is extremely important that you do not stop taking Tenofovir GH without your doctor’s advice. If you have Hepatitis B infection or HIV and HBV infection together, you may have a “flare-up” of Hepatitis B if you stop taking Tenofovir GH, where the disease suddenly returns in a worse way than before. This flare-up may lead to liver failure and possibly liver transplantation or death
After stopping Tenofovir GH, tell your doctor immediately about any new, Tenofovir GH unusual, or worsening symptoms that you notice after stopping treatment.
After you stop taking Tenofovir GH, your doctor will still need to check your health and take blood tests to check your liver for several months.
There have been other side effects in patients taking Tenofovir GH.
However, these side effects may have been due to other medicines that patients were taking or to the illness itself.
Some of these side effects can be serious.
This list of side effects is not complete.
If you have questions about side effects, ask your doctor or pharmacist.
You should report any new or continuing symptoms to your doctor or pharmacist right away.
Your doctor or pharmacist may be able to help you manage these side effects.
After taking Tenofovir GH
Storage
Keep Tenofovir GH and all other medications out of reach of children.
A locked cupboard at least one-and-a half metres above the ground is a good place to store them.
Store Tenofovir GH at room temperature (below 25°C).
It should remain stable until the expiration date printed on the label.
Do not keep your medicine in places that are too hot or cold.
Do not leave Tenofovir GH in the car or on a window sill.
Heat and dampness can destroy some medicines.
Keep your Tenofovir GH tablets in the bottle with the cap tightly closed until you take them.
If you take Tenofovir GH tablets out of their pack they may not keep well.
Do not keep medicine that is out of date or that you no longer need.
If you throw any medicines away make sure that children will not find them.
General Advice
Talk to your doctor or pharmacist if you have any questions about this medicine or your condition.
Medicines are sometimes prescribed for purposes other than those listed in a Consumer Medicine Information.
If you have any concerns about this medicine, ask your doctor or pharmacist.
Your doctor or pharmacist can give you information about this medicine that was written for doctors or pharmacists (Product Information/Data Sheet).
Do not use this medicine for a condition for which it was not prescribed.
Do not share this medicine with other people.
Do not use if seal over bottle opening is broken or missing.
Product description
What it looks like
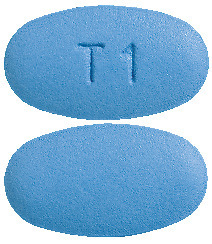
Tenofovir GH 291 mg film coated tablets are blue coloured, oval shaped, biconvex film-coated tablets debossed (indented) on one side with “T1” and plain on other side.
Tenofovir GH 291 mg tablets are supplied in bottles containing 30 or 100 tablets with a child resistant closure.
Note: Not all pack sizes may be marketed.
Ingredients
Active ingredient
Each Tenofovir GH tablet contains 291 mg tenofovir disoproxil phosphate as active ingredient.
Note: The innovator product contains 300 mg of tenofovir disoproxil fumarate. 291 mg of tenofovir disoproxil phosphate is equivalent to 300 mg of tenofovir disoproxil fumarate. Bioequivalence has been established between the two salt forms of tenofovir disoproxil.
Other ingredients
- croscarmellose sodium;
- stearic acid;
- microcrystalline cellulose;
- OPADRY II complete film coating system 39K505001 Blue (ARTG 138537).
Australian Registration Numbers
Tenofovir GH 291 mg:
AUST R 269193.
Distributor
Generic Health Pty Ltd
Suite 2, Level 2
19-23 Prospect Street
Box Hill, VIC, 3128
Australia
Email: ghinfo@generichealth.com.au
Telephone: +61 3 9809 7900
Website: www.generichealth.com.au
This leaflet was prepared in January 2025.