What is in this leaflet
This leaflet answers some common questions about Tracleer.
It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you using this medicine against the benefits they expect it will have for you.
If you have any concerns about using this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine.
You may need to read it again.
What Tracleer is used for
Tracleer (TRAK-leer) is used for the treatment of high blood pressure in the blood vessels between the heart and the lungs. This condition is called pulmonary arterial hypertension.
This medicine acts to reduce abnormally high blood pressure by widening these blood vessels. It belongs to the class of medicines known as endothelin receptor antagonists.
Your doctor however, may prescribe Tracleer for another purpose.
Ask your doctor if you have any questions why it has been prescribed for you.
This medicine is only available with a doctor's prescription.
Before you take Tracleer
When you must not take Tracleer
Do not take this medicine if you are:
• pregnant or intend to become pregnant. You must stop taking the medicine at least 3 months before trying to become pregnant.
It is known that this medicine causes harm to the developing baby if you take it during pregnancy and in the three months before becoming pregnant.
- breastfeeding:
Tell your doctor immediately if you are breastfeeding. You are advised to stop breastfeeding if this medicine is prescribed for you because it has been reported that this drug passes into the milk in a woman who was taking this medicine.
• being treated with cyclosporine A (a medicine used after a transplant or to treat psoriasis)
• being treated with glibenclamide (a medicine used for diabetes)
Do not take Tracleer if you are allergic to it or any of the ingredients listed at the end of this leaflet.
Do not take Tracleer if you have moderate to severe liver disorder.
Do not take it after the expiry date (EXP) printed on the pack.
If you take it after the expiry date has passed, it may not work as well.
Do not take it if the packaging is torn or shows signs of tampering.
Before you start to take Tracleer
Tracleer may harm sperm. All men should use effective birth control while taking this medicine and for 3 months after they stop taking it.
If sexually active, you must use a hormonal and barrier method of contraception.
This medicine may reduce the effectiveness of hormone contraceptives such as the pill and hormone patches, implants or injection. It is important to use other contraceptives, like condoms or an intrauterine device.
Your doctor will advise you about using reliable contraceptives before taking this medicine.
You must have a negative pregnancy test at the time of starting treatment if you are sexually active.
Your doctor will need evidence that you are not pregnant.
Tell your doctor if:
1. you are a woman of childbearing potential and not using reliable contraceptive methods. You must have a negative pregnancy test before beginning treatment. The test should be performed on the second day of a normal menstrual period or 11 days after the last unprotected sexual intercourse, whichever is later. Your doctor will advise you about using reliable contraception before taking or whilst taking this medicine.
Hormonal contraception on its own is not a reliable option because this medicine may make this method ineffective in preventing pregnancy. Hormonal contraceptives include ones you take orally (the pill), patches you put on your skin, ones that are injected and implants. You should ALWAYS use additional methods of contraception such as condoms and IUDs and not rely just on hormonal contraception. You should have a pregnancy test every month while you are taking this medicine. You must stop taking this medicine for at least 3 months prior to becoming pregnant.
2. you are breastfeeding or planning to breastfeed.
It is not known whether Tracleer passes into breast milk.
3. you have allergies to:
• any other medicines
• any other substances such as foods, preservatives or dyes.
4. you have or have had any medical conditions, especially the following:
• pulmonary arterial hypertension or lung disease/ condition
• anaemia
• hypotension
• liver or renal disorders
• heart failure or disease
• HIV infection
If you have not told your doctor about any of the above, tell them before you use Tracleer.
While taking Tracleer
Do not become pregnant while taking this medicine. You must have a pregnancy test every month while you are taking this medicine.
If there is any delay in getting period or any other reason to suspect pregnancy, you must tell your doctor immediately for pregnancy testing. Tracleer may harm unborn babies.
Your doctor will need evidence that you are not pregnant before prescribing this medicine again.
Your doctor may arrange for regular blood tests to check for changes in your liver function and haemoglobin level.
Interactions with other medicines
Tell your doctor if you are taking any other medicines including any that you buy without a prescription from your pharmacy, supermarket or health food shop.
Some medicines may interfere with Tracleer. These include:
- hormonal contraceptives (oral, injectable, transdermal and implantable)
- simvastatin, medicines for lowering blood fats
- medicines for diabetes such as glibenclamide and tolbutamide
- medicines for fungal infections such as ketoconazole, itraconazole, fluconazole and voriconazole
- medicines for bacterial infections such as rifampicin
- medicine to prevent organ transplantation rejection such as cyclosporine A, tacrolimus and sirolimus
- medicines for rheumatoid arthritis or psoriasis / dermatitis
- lopinavir+ritonavir or other ritonavir-boosted protease inhibitors and nevirapine (used to treat HIV);
- warfarin (used as anti-clotting medicines)
- digoxin (used to treat heart rhythm disorders)
- sildenafil or tadalafil (used to treat erectile dysfunction and/or pulmonary arterial hypertension)
- phenytoin, carbamazepine or phenobarbital (medicines for seizures)
- nimodipine
- St John's Wort
These medicines may be affected by Tracleer or may affect how well it works. You may need to use different amounts of your medicines, or take different medicines.
Your doctor will advise you.
Your doctor or pharmacist has more information on medicines to be careful with or to avoid while taking Tracleer.
How to take Tracleer
How much to take
Always take this medicine exactly as your doctor has instructed you.
You should check with your doctor or pharmacist if you are unsure.
Adults
The usual dose is one tablet, twice daily. For the first 4 weeks you will take a 62.5 mg tablet twice daily.
Depending on how you respond to the medicine, your doctor may increase the dosage after four weeks to a 125 mg tablet twice daily.
Patients with low body weight
If you have body weight less than 40 kg but are over 12 years old, your doctor may advise you take a 62.5 mg tablet twice daily.
Children aged 3 years and over
The dose will be determined by your doctor depending on the weight of the child.
If you do not think the medicine is working or you think it is working too well, talk to your doctor. Your doctor may need to change the dose you are taking.
How to take this medicine
Tracleer is taken by mouth, in the morning and evening, and can be taken with or without food.
How long to take it
Do not stop taking this medicine unless your doctor tells you to.
Stopping your treatment may lead to a worsening of your symptoms. Your doctor may tell you to reduce the dose over a few days before stopping completely.
If you forget to take it
If it is almost time for your next dose, skip the dose you missed and take the next dose when you are meant to.
Do not take a double dose to make up for the dose you have missed.
This may increase the chance of getting an unwanted side effect.
If there is still a long time to go before your next dose, take it as soon as you remember and then go back to taking it as you would normally.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering when to take your medicine, ask your pharmacist for hints.
If you take too much (overdose)
Immediately telephone your doctor or the Poisons Information Centre or go to Accident and Emergency at your nearest hospital if you think you or anyone else may have taken too much Tracleer.
Do this even if there are no signs of discomfort or poisoning.
Poison Information Centre telephone numbers:
- Australia: 13 11 26
- New Zealand: 0800 POISON or 0800 764 766
You may need urgent medical attention.
While you are using Tracleer
Things you must do
It is very important that you have a liver function blood test before you start treatment and every month after that.
Tracleer can cause liver damage if it is not found early. Because this side effect may not cause symptoms at first, only a blood test can show that you have early liver damage. Regular blood tests let your doctor change or stop your therapy before there is permanent damage.
You should have a blood test for anaemia after 1 and 3 months, and then every 3 months for the rest of your treatment.
You need to have pregnancy tests monthly if you are a female of childbearing age
Things you must not do
Do not give the tablets to anyone else even if they have the same symptoms as you.
Things to be careful of
If you feel dizzy or that your vision is blurred whilst taking this medicine, do not drive or operate any tools or machinery.
Side Effects
Tell your doctor immediately or go to Accident and Emergency at your nearest hospital if you notice any of the following:
- shortness of breath or difficulty in breathing
- anaphylaxis and/ or swelling, most commonly around the eyes, lips, tongue or throat
- nausea
- vomiting
- fever
- unusual tiredness
- stomach pain
- yellowing of your skin or the whites of your eyes (jaundice)
- dark-coloured urine
- chest pain
These may be serious side effects of Tracleer. You may need urgent medical attention.
Serious side effects are uncommon.
Other side effects of this medicine include:
- headache
- respiratory tract infection
- dizziness or fainting
- anaemia
- abnormal liver function test or liver disorders
- inflamed throat and irritated nasal passages
- flushing (hot flashes)
- swelling of ankle, leg and joint or other signs of fluid retention
- joint pain
- low blood pressure
- blood disorders
- fast heart beat
- tiredness
- hypersensitivity reactions including itching, rash and skin inflammation
- blocked or runny nose
- heartburn or acid reflux
- diarrhoea
- redness of the skin
- blurred vision
- nosebleed
- body ache and pain
- worsening of existing lung disease
See your doctor if any of these worry you.
Tell your doctor if you notice anything else that is making you feel unwell.
Other side effects not listed above may occur in some consumers.
Do not be alarmed by this list of possible side effects.
You may not experience any of them.
After using Tracleer
Storage
Store this medicine in the original bottle, in a cool, dry place where the temperature stays below 25°C.
Keep out of reach of children.
Disposal
Medicines should not be disposed of in wastewater or household waste.
Ask your pharmacist how to dispose of medicines you no longer require. These measures will help to protect the environment.
Product description
What it looks like
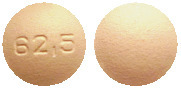
Tracleer 62.5 mg tablets are orange-white, round tablets, embossed with "62.5" on one side.
Tracleer 125 mg tablets are orange-white, oval tablets, embossed with "125" on one side.
Pack size
Bottles of 60 tablets.
Ingredients
Active ingredient:
Tracleer 62.5 mg: 62.5 mg bosentan (as monohydrate) per tablet.
Tracleer 125 mg: 125 mg bosentan (as monohydrate) per tablet.
Inactive ingredients:
- maize starch
- pregelatinised maize starch
- sodium starch glycollate
- povidone
- glyceryl behenate
- magnesium stearate
- hypromellose
- triacetin
- talc
- titanium dioxide
- iron oxide yellow
- iron oxide red
- ethylcellulose.
Sponsor
JANSSEN-CILAG Pty Ltd
1-5 Khartoum Road
Macquarie Park NSW 2113 Australia
Telephone: 1800 226 334
NZ Office: Auckland New Zealand
Telephone: 0800 800 806
This leaflet was prepared in January 2024.
Australian Register Number:
Tracleer 62.5 mg - AUST R 91919
Tracleer 125 mg - AUST R 91920