TREXJECT®
| Consumer Medicine Information (CMI) summary |
The full CMI on the next page has more details. If you are worried about using this medicine, speak to your doctor or pharmacist.
WARNING: Important safety information is provided in a boxed warning in the full CMI. Read before using this medicine.
| 1. Why am I using TREXJECT? |
TREXJECT contains the active ingredient methotrexate (as sodium). TREXJECT is used to treat inflammatory conditions, including severe psoriasis, and severe rheumatoid arthritis.
For more information, see Section 1. Why am I using TREXJECT? in the full CMI.
| 2. What should I know before I use TREXJECT? |
Do not use if you have ever had an allergic reaction to TREXJECT or any of the ingredients listed at the end of the CMI.
Talk to your doctor if you have any other medical conditions, take any other medicines, or are pregnant or plan to become pregnant or are breastfeeding.
For more information, see Section 2. What should I know before I use TREXJECT? in the full CMI.
| 3. What if I am taking other medicines? |
Some medicines may interfere with TREXJECT and affect how it works.
A list of these medicines is in Section 3. What if I am taking other medicines? in the full CMI.
| 4. How do I use TREXJECT |
- TREXJECT is given as a once weekly injection under the skin (subcutaneous)
- You may decide together with your doctor on a suitable weekday each week to receive your injection
More instructions can be found in Section 4. How do I use TREXJECT? in the full CMI
| 5. What should I know while using TREXJECT? |
| Things you should do |
|
| Things you should not do |
|
| Driving or using machines |
|
| Drinking alcohol |
|
| Looking after your medicine |
|
For more information, see Section 5. What should I know while using TREXJECT? in the full CMI.
| 6. Are there any side effects? |
Side effects that require urgent medical attention include: Signs of an allergic reaction, such as chest tightness, difficulty breathing, swelling of face lips and tongue, rash; signs suggesting a blood disorder, such as persistent fever, bruising, bleeding, paleness, blood in stools, urine, or vomit; pinpoint red spots or painful blistering resulting in peeling of layers of skin, severe blisters, signs of infection such as fever, chills, sore throat, signs of liver problems such as yellowing of skin, eyes, loss of appetite, itching and dark coloured urine, chest pain, fits; signs of lung damage, such as a dry non-productive cough or shortness of breath.
For more information, including what to do if you have any side effects, see Section 6. Are there any side effects? in the full CMI.
| WARNING Use of TREXJECT is only for severe disease which is not treated adequately by other forms of therapy and only when diagnosis has been made. Due to the risk of fatal or severe reactions to methotrexate, you should be treated under supervision of your doctor or specialist. Methotrexate can cause severe damage to blood, liver, kidneys, lungs and gastrointestinal system, and may increase your risk of bleeding. This is more likely when methotrexate is used at high or repeated doses. The risk of damage to these body systems will depend on several factors, including your health before starting methotrexate, and other medicines that you take. Serious and potentially fatal infections may occur with methotrexate therapy. Your doctor will monitor your blood counts, liver function and kidney function while you are using methotrexate. Tell your doctor immediately if you experience diarrhoea or stomach ulcers, as methotrexate may need to be paused to prevent further bleeding. If you notice symptoms such as a dry non-productive cough or shortness of breath, stop using methotrexate and talk to your doctor. Avoid alcohol while using methotrexate. Tell your doctor if you are taking nonsteroidal anti-inflammatory drugs (NSAIDs), which are medicines used to relieve pain, swelling and inflammation. Use of methotrexate to treat severe rheumatoid arthritis and psoriasis in children has not been well documented. Methotrexate has caused foetal death and/or congenital anomalies. It should not be used in pregnant women or in those who might become pregnant. Methotrexate should not be used in the treatment of psoriasis and rheumatoid arthritis in pregnant women. Methotrexate should not be started until it is confirmed you are not pregnant, and due to the serious risk to the foetus you should not become pregnant while undergoing treatment. Pregnancy should be avoided if either partner is receiving methotrexate, during and for a minimum of 6 months after therapy has ceased. TREXJECT should be used once a week on the same day each week. Taking TREXJECT more frequently than once each week may cause life-threatening or fatal toxicity. If you are unsure of how you should take TREXJECT, talk to your doctor or pharmacist. |
TREXJECT®
Active ingredient(s): methotrexate (as sodium)
| Consumer Medicine Information (CMI) |
This leaflet provides important information about using TREXJECT. You should also speak to your doctor or pharmacist if you would like further information or if you have any concerns or questions about using TREXJECT.
Where to find information in this leaflet:
1. Why am I using TREXJECT?
2. What should I know before I use TREXJECT?
3. What if I am taking other medicines?
4. How do I use TREXJECT?
5. What should I know while using TREXJECT?
6. Are there any side effects?
7. Product details
| 1. Why am I using TREXJECT? |
TREXJECT contains the active ingredient methotrexate (as sodium). Methotrexate belongs to a group of medicines known as immunosuppressants. Methotrexate works by preventing the growth of certain cells.
TREXJECT is used to treat:
- Severe Psoriasis (a skin disease with thickened patches of red skin, often with silver scales)
- Severe Rheumatoid Arthritis (a disease mainly affecting the joints with pain and swelling)
| 2. What should I know before I use TREXJECT? |
Warnings
Do not use TREXJECT if you:
- are allergic to methotrexate, or any of the ingredients listed at the end of this leaflet
- have liver disease or poor liver function
- are an alcoholic
- have kidney disease or poor kidney function
- have any blood disorders, or conditions which cause a reduced number of red blood cells, white blood cells or platelets
- have problems with your immune system such as severe or repeated infections, e.g. tuberculosis or HIV
- have an infection
- have a stomach ulcer or ulcerative colitis (bleeding from your bowel)
- are pregnant, or if you or your partner are planning to get pregnant
- are breastfeeding
- have poor nutritional status, such as malnutrition
- are about to have surgery that involves general anaesthetic
- are taking a retinoid such as acitretin (a medicine used to treat skin conditions including psoriasis)
Do not receive a live vaccine while you are using TREXJECT.
Check with your doctor if you:
- have any sort of infection or immune system disorder e.g. sinusitis, tooth abscess etc.
- have a stomach ulcer or ulcerative colitis (bleeding from the bowel)
- have fluid or swelling in your abdomen or stomach
- have fever, chills, sore throat or mouth ulcers
- have any increased or unusual bleeding or bruising
- have kidney problems
- have lung problems
- have diabetes
- have a folate deficiency
- are experiencing tiredness, headaches, shortness of breath, dizziness, or if you are pale
- are receiving radiotherapy e.g. X-rays, ultraviolet radiotherapy
During treatment, you may be at risk of developing certain side effects. It is important you understand these risks and how to monitor for them. See additional information under Section 6. Are there any side effects?
Pregnancy and breastfeeding
Check with your doctor if you are pregnant, or you or your partner intend to become pregnant.
If you wish to become pregnant you should consult your doctor, who may refer you for specialist advice before the planned start of treatment.
You and your partner must avoid becoming pregnant, and use a reliable method of contraception during, and for at least 6 months after treatment with TREXJECT.
The possibility of pregnancy must be excluded e.g. pregnancy test prior to starting treatment with TREXJECT.
TREXJECT passes into breast milk. Stop breast-feeding prior to, and during treatment with TREXJECT.
Use in liver impairment
- Where possible, it is recommended that TREXJECT is not used where there is impaired liver function, or liver disorders, including where alcoholism is present.
Use in kidney impairment
- Where possible, it is recommended that TREXJECT is not used where there is decreased kidney function.
Use in the elderly
- Elderly patients are more likely to have decreased liver and kidney function, and decreased folate levels. Lower doses may need to be considered, and increased monitoring may be required.
Monitoring and follow up
To check you are receiving the correct dose, and to detect any severe side effects, your doctor may carry out checkups and laboratory tests from time to time. It is very important that you appear for these scheduled tests. If the results of any of these tests are conspicuous, your doctor will adjust your treatment accordingly.
These tests may include:
- examinations of the mouth and throat for any changes in the mucosa
- blood tests/ blood count with number of blood cells
- blood tests to monitor liver function
- imaging tests to monitor liver condition
- small sample of tissue taken from the liver in order to examine it more closely (biopsy)
- blood tests to monitor kidney function
- lung function tests and respiratory tract monitoring
If you, your partner or your caregiver notice new onset or worsening of neurological symptoms including general muscle weakness, disturbance of vision, changes in thinking, memory and orientation leading to confusion and personality changes contact your doctor immediately because these may be symptoms of a very rare, serious brain infection called progressive multifocal leukoencephalopathy (PML).
| 3. What if I am taking other medicines? |
Tell your doctor or pharmacist if you are taking any other medicines, including any medicines, vitamins, or supplements that you buy without a prescription from your pharmacy, supermarket or health food shop.
The concurrent use of TREXJECT and some other medicines may interfere with each other, including (but not limited to):
- retinoids such as acitretin (a medicine used to treat skin conditions including psoriasis)
- medicines for gastric reflux such as omeprazole and pantoprazole
- anticancer drugs such as cisplatin, mercaptopurine or asparaginase
- antibiotics such as trimethoprim, tetracyclines and sulphonamides
- non-steroidal anti-inflammatory drugs or salicylates (medicines used to relieve pain, swelling and inflammation such as aspirin, diclofenac and ibuprofen)
- medicines for epilepsy such as phenytoin
- corticosteroids such as hydrocortisone and prednisone
- medicines for diabetes such as sulphonylureas
- medicines that reduce cholesterol such as cholestyramine
- medicines for gout such as allopurinol and probenecid
- vitamin preparations that contain folic acid
- other medicines for rheumatoid arthritis, such as leflunomide and sulfasalazine
- other medicines for psoriasis such as etretinate
- medicines for heart problems such as amiodarone
- medicines used to treat asthma and related compounds such as theophylline
- antimalarial medicine such as pyrimethamine
- other immunosuppressive medication
TREXJECT may also be affected by, or interfere with, the following:
- blood transfusions
- nitrous oxide anaesthetics
- vaccinations
- alcohol
- radiation e.g. X-rays, radiotherapy
Check with your doctor or pharmacist if you are not sure about what medicines, vitamins or supplements you are taking and if these affect TREXJECT.
| 4. How do I use TREXJECT? |
How much to use
- Your doctor will decide what dose you should be given, when it should be given, and for how long you will receive it. This depends on your condition and other factors, such as your weight, age, blood tests, how well your kidneys and liver are working, and whether other medicines are being given at the same time.
- Response to TREXJECT treatment is usually seen after 4-8 weeks.
- Always use this medicine exactly as your doctor has told you. Check with your doctor or pharmacist if you are not sure.
- Too much TREXJECT may be fatal.
When to use TREXJECT
- TREXJECT is given as a once weekly injection under the skin (subcutaneous) for the treatment of severe arthritis and severe psoriasis. You may decide together with your doctor on a suitable weekday each week to receive your injection.
How to use TREXJECT
- TREXJECT is usually given by a doctor or nurse as a once weekly injection under the skin.
- Your doctor may decide that you can administer the injection yourself under the skin (subcutaneously). If you will be self-administering TREXJECT, your doctor or nurse will give you detailed instructions on how to do this. Information is also provided below on how to self-administer the injection.
The following protective recommendations are given due to the toxic nature of this substance:
- Personnel should be trained in good handling technique.
- Pregnant staff should not handle or administer this drug.
- It is important that you and your caregiver wear disposable gloves when handling methotrexate injection.
- Accidental contact with the skin or eyes should be treated immediately by copious lavage with water or sodium bicarbonate solution; medical attention should be sought.
Carefully read the instructions below before starting your injection, and always use the injection technique advised by your doctor, pharmacist or nurse.
Preparation:
Select a clean, well-lit and flat working surface. Wash your hands carefully. Unpack the methotrexate prefilled syringe and read the package leaflet carefully.
Remove the pre-filled syringe from the packaging at room temperature. Before use, check the TREXJECT syringe for visual defects (or cracks).
Injection site:
The best sites for injection are the:
- upper thighs, and
- abdomen except around the navel
However, obese patients (i.e. patients with a body weight of more than 100 kg) should inject TREXJECT solely in the upper thighs (not in the abdomen).
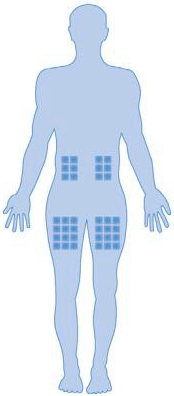
If someone is helping you with the injection, he/she may also give the injection into the back of your arms, just below the shoulder.
Change the injection site with each injection. This may reduce the risk of developing irritations at the injection site.
Never inject into skin that is tender, bruised, red, hard, scarred or where you have stretch marks. If you have psoriasis, you should avoid injecting directly into any raised, thick, red or scaly skin patches or lesions.
Injecting the solution:
- Remove the pre-filled syringe from the packaging at room temperature
- Warm the syringe gently in your hands before injecting to avoid pain
- Choose an injection site and clean the area
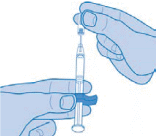
- Carefully remove the grey protective plastic cap by pulling it straight off the syringe. If the cap is very stiff, turn it slightly with a pulling movement
Important: Do not touch the needle of the pre-filled syringe.
Note: Once you have removed the cap, perform the injection without delay.
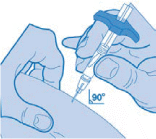
- Using two fingers, pinch up a fold of skin and quickly insert the needle into the skin at a 90-degree angle
- Insert the needle fully into the fold of skin. Push the plunger down completely to inject the liquid underneath your skin. Hold the skin securely until the injection is completed. Carefully pull the needle straight out
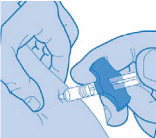
Methotrexate should not come into contact with the surface of the skin or mucosa. In the event of contamination, the affected area must be rinsed immediately with plenty of water.
If you or someone around you is injured by the needle, consult your doctor immediately and do not use this pre-filled syringe.
Disposal and other handling:
Keep your syringes and syringe disposal unit out of reach of children; lock the supplies away if possible.
Never re-use syringes or needles.
Always use a sterile (aseptic) technique as described here. If in any doubt, discard needles, syringes or solution and start again.
Always place the used syringes in the appropriate disposal unit.
For further information of administration of TREXJECT, please visit www.trexject.com.au
If you forget to use TREXJECT
TREXJECT should be used once weekly at the same time each week. If you miss your dose at the usual time, do it as soon as you remember and then follow on with the next injection one week later.
If it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to.
Do not take a double dose to make up for the dose you missed.
Ask your doctor if you are not sure what to do or have trouble remembering to inject your medicine.
If you use too much TREXJECT
If you think that you have used too much TREXJECT you may need urgent medical attention.
You should immediately:
- phone the Poisons Information Centre
(by calling 13 11 26), or - contact your doctor, or
- go to the Emergency Department at your nearest hospital.
You should do this even if there are no signs of discomfort or poisoning.
| 5. What should I know while using TREXJECT? |
Driving or using machines
Do not drive or operate machinery until you know how methotrexate affects you. Methotrexate may cause dizziness or tiredness in some people and therefore may affect alertness
Drinking alcohol
Avoid drinking alcohol while you are being treated with TREXJECT, as this may cause permanent liver damage
Looking after your medicine
Follow the instructions in the carton on how to take care of your medicine properly.
Store below 25°C in a cool dry place away from moisture, heat or sunlight; for example, do not store it:
- in the bathroom or near a sink, or
- in the car or on windowsills.
Keep it where young children cannot reach it.
When to discard your medicine
TREXJECT is a single use injection for use in one patient on one occasion only. Once opened, use immediately.
Getting rid of any unwanted medicine
TREXJECT should NOT be placed in your household recycling bin, waste bin or public litter bin.
Always make sure your used TREXJECT syringes are secured in a strong plastic container, or a sharps bin before returning to your public hospital or participating pharmacy for disposal in a cytotoxic waste bin.
You should talk to your doctor, pharmacist or local council regarding disposal of these items.
Do not use this medicine after the expiry date.
| 6. Are there any side effects? |
All medicines can have side effects. If you do experience any side effects, most of them are minor and temporary. However, some side effects may need medical attention.
See the information below and, if you need to, ask your doctor or pharmacist if you have any further questions about side effects.
Less serious side effects
| Less serious side effects | What to do |
Gut and digestion:
| Speak to your doctor if you have any of these less serious side effects and they worry you. |
Serious side effects
| Serious side effects | What to do |
Gut and digestion:
| Call your doctor straight away, or go straight to the Emergency Department at your nearest hospital if you notice any of these serious side effects. |
Tell your doctor or pharmacist if you notice anything else that may be making you feel unwell.
Other side effects not listed here may occur in some people.
Reporting side effects
After you have received medical advice for any side effects you experience, you can report side effects to the Therapeutic Goods Administration online at www.tga.gov.au/reporting-problems. By reporting side effects, you can help provide more information on the safety of this medicine.
Always make sure you speak to your doctor or pharmacist before you decide to stop taking any of your medicines.
| 7. Product details |
This medicine is only available with a doctor's prescription.
What TREXJECT contains
| Active ingredient (main ingredient) | methotrexate (as sodium) |
| Other ingredients (inactive ingredients) | sodium chloride sodium hydroxide water for injections |
Do not take this medicine if you are allergic to any of these ingredients.
What TREXJECT looks like
TREXJECT is a clear yellow-brown solution in a glass pre-filled syringe, packed in blisters. TREXJECT is available in the following presentations:
- Syringe with 0.15 mL solution for injection, equivalent to 7.5 mg methotrexate. The syringe barrel is made of glass and has a red colour coded plastic backstop. (AUST R 233714)
- Syringe with 0.20 mL solution for injection, equivalent to 10 mg methotrexate. The syringe barrel is made of glass and has a green colour coded plastic backstop. (AUST R 233715)
- Syringe with 0.30 mL solution for injection, equivalent to 15 mg methotrexate. The syringe barrel is made of glass and has a purple colour coded plastic backstop. (AUST R 233717)
- Syringe with 0.40 mL solution for injection, equivalent to 20 mg methotrexate. The syringe barrel is made of glass and has a dark red colour coded plastic backstop. (AUST R 233719)
- Syringe with 0.50 mL solution for injection, equivalent to 25 mg methotrexate. The syringe barrel is made of glass and has a blue colour coded plastic backstop. (AUST R 233721)
You may notice an air bubble in the pre-filled syringe. The air bubble in the syringe guarantees dispensing of the full dose from the syringe and does not need to be removed. It will dissolve under the skin.
Who distributes TREXJECT
Link Medical Products Pty Ltd
5 Apollo Street
Warriewood NSW 2102
This leaflet was updated in April 2022.
Published by MIMS October 2022
