1 Name of Medicine
Elexacaftor, tezacaftor and ivacaftor in combination, and ivacaftor.
2 Qualitative and Quantitative Composition
Trikafta 100/50/75. One morning dose film-coated tablet contains elexacaftor 100 mg, tezacaftor 50 mg and ivacaftor 75 mg.
One evening dose film-coated tablet contains ivacaftor 150 mg.
Excipients with known effect: sugars as lactose (150 mg ivacaftor tablet).
For the full list of excipients, see Section 6.1 List of Excipients.
Trikafta 50/25/37.5. One morning dose film-coated tablet contains elexacaftor 50 mg, tezacaftor 25 mg and ivacaftor 37.5 mg.
One evening dose film-coated tablet contains ivacaftor 75 mg.
Excipients with known effect: sugars as lactose (75 mg ivacaftor tablet).
For the full list of excipients, see Section 6.1 List of Excipients.
3 Pharmaceutical Form
Composite pack. Trikafta 100/50/75. Elexacaftor/tezacaftor/ivacaftor 100 mg/50 mg/75 mg tablet. Orange, capsule shaped tablet with "T100" debossed on one side and plain on the other (7.9 mm x 15.5 mm).
Ivacaftor 150 mg tablet. Light blue, capsule-shaped tablet printed with "V 150" in black ink on one side and plain on the other (16.5 mm x 8.4 mm).
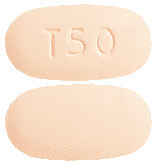
Trikafta 50/25/37.5. Elexacaftor/tezacaftor/ivacaftor 50 mg/25 mg/37.5 mg tablet. Light orange, capsule shaped tablet with "T50" debossed on one side and plain on the other (6.4 mm x 12.2 mm).
Ivacaftor 75 mg tablet. Light blue, capsule shaped tablet printed with "V 75" in black ink on one side and plain on the other (12.7 mm x 6.8 mm).
4 Clinical Particulars
4.9 Overdose
No specific antidote is available for overdose with Trikafta. Treatment of overdose consists of general supportive measures including monitoring of vital signs and observation of the clinical status of the patient.
For information on the management of overdose, contact the Poisons Information Centre on 131126 (Australia).
5 Pharmacological Properties
5.3 Preclinical Safety Data
Genotoxicity. Elexacaftor, tezacaftor and ivacaftor were all negative for genotoxicity in the following assays: Ames test for bacterial gene mutation, in vitro chromosomal aberration assay (in TK6 [human lymphoblastoid] cells for elexacaftor, and in Chinese hamster ovary cells for tezacaftor and ivacaftor), and in vivo bone marrow micronucleus test (performed in rats with elexacaftor, and in mice for tezacaftor and ivacaftor).
Carcinogenicity. Elexacaftor was not carcinogenic in a 6-month study in transgenic (Tg.rasH2) mice, involving oral administration at doses up to 50 mg/kg/day (yielding systemic exposure 8-fold higher than in patients at the MRHD based on summed AUCs for elexacaftor and M23-ELX).
No evidence of tumourigenicity by tezacaftor was observed in a 6-month study in transgenic (Tg.rasH2) mice and in a conventional 2-year study in rats, conducted by the oral route. The highest doses tested (500 mg/kg/day in mice, 50 mg/kg/day in male rats and 75 mg/kg/day in female rats) yielded exposure to tezacaftor and its M1 and M2 metabolites that was 1.5-fold higher in mice, 1.2-fold higher in male rats, and 2.1-fold higher in female rats than in patients at the MRHD (based on summed AUCs).
Two-year oral studies in mice and rats demonstrated that ivacaftor was not carcinogenic in either species. Plasma exposures to ivacaftor in mice at the non-carcinogenic dosage (200 mg/kg/day, the highest dosage tested) were approximately 5- to 9-fold higher than the plasma levels measured in humans following Trikafta therapy, and at least 1.1- to 2.3-fold higher with respect to the summed AUC for ivacaftor and its major metabolites. Plasma exposures to ivacaftor in rats at the non-carcinogenic dosage (50 mg/kg/day, the highest dosage tested) were approximately 20- to 36-fold higher than the plasma levels measured in humans following Trikafta therapy, and 6- to 9-fold higher with respect to the summed AUC for ivacaftor and its major metabolites.
6 Pharmaceutical Particulars
6.7 Physicochemical Properties
Chemical structure.
https://stagingapi.mims.com/au/public/v2/images/fullchemgif/CSELEXAC.gif Elexacaftor: N-(1,3-dimethyl-1H-pyrazole-4-sulfonyl)-6-[3-(3,3,3-trifluoro-2,2-dimethylpropoxy)-1H-pyrazol-1-yl]-2-[(4S)-2,2,4-trimethylpyrrolidin-1-yl]pyridine-3-carboxamide.
https://stagingapi.mims.com/au/public/v2/images/fullchemgif/CSTEZCAF.gif Tezacaftor: 1-(2,2-difluoro-2H-1,3-benzodioxol-5-yl)-N-{1-[(2R)-2,3-dihydroxypropyl]-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)-1Hindol-5-yl}cyclopropane-1-carboxamide.
https://stagingapi.mims.com/au/public/v2/images/fullchemgif/CSIVACAF.gif Ivacaftor: N-(2,4-di-tert-butyl-5-hydroxyphenyl)-4-oxo- 1,4-dihydroquinoline-3-carboxamide.
CAS number. Elexacaftor: 2216712-66-0.
Tezacaftor: 1152311-62-0.
Ivacaftor: 873054-44-5.
7 Medicine Schedule (Poisons Standard)
Schedule 4.
Summary Table of Changes
https://stagingapi.mims.com/au/public/v2/images/fulltablegif/TRIKAFST.gif