What is in this leaflet
What Vagifem® Low is used for
Before you use Vagifem® Low
How to use Vagifem® Low
While you are using Vagifem® Low
Side effects
Storage
Product Description
Directions for Use
This leaflet answers some common questions about Vagifem® Low. It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you using Vagifem® Low against the benefits they expect it will have for you.
Ask your doctor or pharmacist if you have any concerns about using this medicine.
Keep this leaflet with the medicine. You may need to read it again.
Vagifem® Low is available only by prescription at pharmacies.
What Vagifem® Low is used for
Vagifem® Low is a local hormone replacement therapy (HRT). Vagifem® Low is a modified release pessary containing the female sex hormone, estradiol. The estradiol in Vagifem® Low is identical to the estradiol produced in the ovaries of women, and is classified as a natural estrogen.
Vagifem® Low is prescribed to treat a condition called atrophic vaginitis. The symptoms include dryness and irritation in the vagina, and pain during sexual intercourse. Atrophic vaginitis is caused by a loss of the female sex hormone, estrogen, which occurs around the menopause.
Vagifem® Low when placed in the vagina allows estradiol to be released. This may relieve discomfort in the vagina.
Your doctor may have prescribed Vagifem® Low for another reason.
Ask your doctor if you have any questions about why Vagifem® Low has been prescribed for you.
Before you use Vagifem® Low
When you must not use it
Do not use Vagifem® Low if:
- you have, or you are suspected of having, or you have ever had, breast cancer
- you have, or you are suspected of having, or you have had, cancer which is sensitive to estrogens, such as cancer of the lining of the womb (endometrium)
- you have any unexplained vaginal bleeding
- you have excessive thickening of the lining of the womb (endometrial hyperplasia) that is not being treated
- you have or have ever had a blood clot in a vein (thrombosis), such as in the legs (deep venous thrombosis) or the lungs (pulmonary embolism)
- you have a blood clotting disorder (such as protein C, protein S or antithrombin deficiency)
- you have or have previously had a disease caused by blood clots in the arteries, such as a heart attack, stroke or angina
- you have or have ever had a liver disease and your liver function tests have not returned to normal
- you have a rare blood problem called ‘porphyria’, which is passed down in families (inherited)
- you are pregnant or suspect you may be pregnant
- you are breast-feeding
- you are allergic to estradiol or any of the other ingredients in Vagifem® Low (listed under ‘Ingredients’)
- it is after the expiry date (‘Expiry’) printed on the pack
- the packaging is torn or shows signs of tampering.
Vagifem® Low should not be used in children or by males.
Stop using your medicine at once and consult your doctor immediately if any of the above conditions appear for the first time while using Vagifem® Low.
Before you start to use it
Medical history and regular check-ups
The use of HRT carries risks which need to be considered when deciding whether to start taking it, or whether to carry on taking it.
Before you start (or restart) HRT, your doctor will ask about your own and your family’s medical history. Your doctor may decide to perform a physical examination. This may include an examination of your breasts and/or internal examination, if necessary. Once you’ve started on Vagifem® Low, you should see your doctor for regular check-ups (3-6 months after starting Vagifem® Low, and at least once a year thereafter).
Go for regular breast screening as recommended by your doctor.
There is only limited experience of treating women older than 65 years’ with Vagifem® Low.
Vaginal infections should be treated before Vagifem® Low are used.
Vagifem® Low, as opposed to systemic estrogen, is for local treatment in the vagina, and the absorption into the blood is low.
Tell your doctor if you have or have ever had any of the following problems before you start the treatment. If so, see your doctor more often for check-ups:
- Asthma
- Epilepsy
- Diabetes
- Gallstones
- High blood pressure
- Migraines or severe headaches
- A liver disorder, such as a benign liver tumour
- Growth of womb lining outside your womb (endometriosis) or a history of excessive growth of the womb lining (endometrial hyperplasia)
- A disease affecting the eardrum and hearing (otosclerosis)
- A disease of the immune system that affects many organs of the body (systemic lupus erythematosus, SLE)
- Increased risk of getting an estrogen-sensitive cancer (such as having a mother, sister or grandmother who has had breast cancer)
- Increased risk of developing blood clots (see ‘Blood clots in a vein (thrombosis)’)
- Fibroids inside your womb
- A very high level of fat in your blood (triglycerides)
- hereditary or acquired angioedema
- Fluid retention due to cardiac or kidney problems.
Any unexplained vaginal bleeding, and persistent or recurrent vaginal bleeding should be examined.
Stop using Vagifem® Low and see a doctor immediately
Stop using Vagifem® Low and see a doctor immediately if you notice any of the following when using HRT:
- Migraine-like headaches which happen for the first time
- Yellowing of your skin or the whites of your eyes (jaundice). These may be signs of a liver disease.
- A large rise in your blood pressure (symptoms may be headache, tiredness, dizziness)
- Any of the conditions mentioned in the ‘When you must not use it’ section
- If you become pregnant
- If you notice signs of a blood clot, such as:
- painful swelling and redness of the legs
- sudden chest pain
- difficulty in breathing.
For more information, see ‘Blood clots in a vein (thrombosis)’.
The following risks apply to hormone replacement therapy (HRT) medicines which circulate in the blood. It is not known how these risks apply to locally administered treatments such as Vagifem® Low.
HRT and cancer
Excessive thickening of the lining of the womb (endometrial hyperplasia) and cancer of the lining of the womb (endometrial cancer)
Taking estrogen-only HRT tablets for a long time can increase the risk of developing cancer of the womb lining (the endometrium). It is uncertain whether long term (more than one year) or repeated use of local vaginally administered estrogen products possess a similar risk.
If you get breakthrough bleeding or spotting, it’s usually nothing to worry about, but you should make an appointment to see your doctor. It could be a sign that your endometrium has become thicker.
Compare
In women who still have a womb and who are not taking HRT, on average, 5 in 1,000 will be diagnosed with endometrial cancer between the ages of 50 and 65.
For women aged 50 to 65 who still have a womb and who take estrogen-only HRT, between 10 and 60 women in 1,000 will be diagnosed with endometrial cancer (i.e. between 5 and 55 extra cases), depending on the dose and for how long it is taken.
Breast cancer
Evidence suggests that using Vagifem® Low does not increase the risk of breast cancer in women who have not had breast cancer in the past. It is not known if Vagifem® Low can be safely used in women who have had breast cancer in the past.
Compare
Women aged 50 to 79 who are not taking HRT, on average, 9 to 17 in 1,000 will be diagnosed with breast cancer over a 5-year period. For women aged 50 to 79 who are taking estrogen-progestagen HRT over 5 years, there will be 13 to 23 cases in 1,000 users (i.e. an extra 4 to 6 cases).
Regularly check your breasts. See your doctor if you notice any changes such as:
- dimpling of the skin
- changes in the nipple
- any lumps you can see or feel.
Additionally, you are advised to join mammography screening programs when offered to you. For mammogram screening, it is important that you inform the nurse/healthcare professional who is actually taking the x-ray that you use HRT, as this medication may increase the density of your breasts which may affect the outcome of the mammogram. Where the density of the breast is increased, mammography may not detect all lumps.
Ovarian cancer
Ovarian cancer is rare - much rarer than breast cancer. The use of estrogen-only or combined estrogen-progestagen HRT has been associated with a slightly increased risk of ovarian cancer.
Compare
The risk of ovarian cancer varies with age. For example, in women aged 50 to 54 who are not taking HRT, about 2 women in 2,000 will be diagnosed with ovarian cancer over a 5-year period. For women who have been taking HRT for 5 years, there will be about 3 cases per 2,000 users (i.e. about 1 extra case).
Effect of HRT on heart and circulation
Blood clots in a vein (thrombosis)
The risk of blood clots in the veins is about 1.3- to 3-times higher in HRT users than in non-users, especially during the first year of taking it.
Blood clots can be serious, and if one travels to the lungs, it can cause chest pain, breathlessness, fainting or even death.
You are more likely to get a blood clot in your veins as you get older and if any of the following applies to you. Inform your doctor if any of these situations applies to you:
- you are unable to walk for a long time because of major surgery, injury or illness
- you are seriously overweight (BMI >30 kg/m²)
- you have any blood clotting problem that needs long-term treatment with a medicine used to prevent blood clots
- if any of your close relatives has ever had a blood clot in the leg, lung or another organ
- you have systemic lupus erythematosus (SLE)
- you have cancer.
For signs of a blood clot, see ‘Stop using Vagifem® Low and see a doctor immediately’.
Compare
Looking at women in their 50s who are not taking HRT, on average, over a 5-year period, 4 to 7 in 1,000 would be expected to get a blood clot in a vein.
For women in their 50s who have been taking estrogen-progestagen HRT for over 5 years, there will be 9 to 12 cases in 1,000 users (i.e. 5 extra cases).
For women in their 50s who have had their womb removed and have been taking estrogen-only HRT for over 5 years, there will be 5 to 8 cases in 1,000 users (i.e. 1 extra case).
Heart disease (heart attack)
There is no evidence that HRT will prevent a heart attack.
Women over the age of 60 years who use estrogen-progestagen HRT are slightly more likely to develop heart disease than those not taking any HRT.
For women who have had their womb removed and are taking estrogen-only therapy there is no increased risk of developing a heart disease.
Stroke
The risk of getting stroke is about 1.5-times higher in HRT users than in non-users. The number of extra cases of stroke due to use of HRT will increase with age.
Compare
Looking at women in their 50s who are not taking HRT, on average, 8 in 1,000 would be expected to have a stroke over a 5-year period. For women in their 50s who are taking HRT, there will be 11 cases in 1,000 users, over 5 years (i.e. 3 extra cases).
Other conditions
HRT will not prevent memory loss. There is some evidence of a higher risk of memory loss in women who start using HRT after the age of 65. Speak to your doctor for advice.
Taking other medicines
Tell your doctor if you are taking any other medicines, including any that you buy without a prescription from your pharmacy, supermarket or health food shop.
Vagifem® Low contains a very small amount of hormone which should not interact with other medicines you are using.
How to use Vagifem® Low
Vagifem® Low is suitable for women who have had their womb removed (have had a hysterectomy), as well as for those who have not.
How to use it
Always use Vagifem® Low exactly as your doctor has instructed you to. Check with your doctor or pharmacist if you are unsure.
You can start treatment with Vagifem® Low on any convenient day. The Vagifem® Low pessary should be inserted into your vagina using the applicator (see the diagrams included in the ‘Directions for Use’ section of this leaflet).
Use one Vagifem® Low pessary each day for the first two weeks, then one pessary twice a week (allowing three to four days between doses e.g. Monday and Friday).
Your doctor will tell you for how long you should use Vagifem® Low.
Vagifem® Low treatment should not affect your normal hygiene routine or lifestyle.
If you forget to use it
If you forget to use Vagifem® Low at the usual time, insert your pessary as soon as you remember. Do not use a double dose to make up for the dose that you have missed.
If you use too much (overdose)
If you have used more Vagifem® Low than you have been prescribed, or have accidentally swallowed Vagifem® Low, contact your doctor or pharmacist for advice.
While you are using Vagifem® Low
You can expect your symptoms to improve within a few weeks of starting Vagifem® Low.
Vagifem® Low can be stopped at any time. You should discuss this with your doctor.
Vagifem® Low is not a contraceptive and will not prevent pregnancy.
If you have any concerns about using Vagifem® Low, ask your doctor or pharmacist. If your doctor tells you to stop using Vagifem® Low, return any unused medicine to your pharmacist.
Used applicators may be returned to the open blister packs and then discarded carefully.
Things you must not do
This medicine is for you only. Do not give it to someone else even if they seem to have the same symptoms as you.
Do not use Vagifem® Low to treat any other complaints unless your doctor tells you to.
Do not change the way you use Vagifem® Low, or change the dosage, without checking with your doctor.
Do not swallow Vagifem® Low. These modified release pessaries are for vaginal use only.
Side effects
All medicines can have side effects. Sometimes they are serious, most of the time they are not.
Tell your doctor or pharmacist if you experience any side effects while you are using Vagifem® Low (whether or not they are mentioned below). You may need medical treatment if you experience some of the side effects.
You may experience the following side effects:
- genital infection with a fungus (thrush)
- headache
- stomach pain
- feeling sick (nausea)
- vaginal bleeding, discharge or discomfort
- rash
- weight increase
- rise in blood pressure
- hot flush.
In very rare cases, unwanted effects that have been reported include:
- breast cancer, or cancer of the lining of the womb
- excessive growth of the lining of the womb
- allergic reaction
- fluid retention (oedema
- swelling under the skin (angioedema)
- depression
- trouble sleeping
- worsening of migraine, where you have had migraines in the past
- blood clots (deep vein thrombosis, DVT)
- diarrhoea
- hives, rash
- itching of the genital area
- vaginal irritation or pain, painful spasm of the vagina or vaginal ulceration
- Vagifem® Low does not treat your symptoms effectively
- weight increase
- increase in blood estrogen (blood test result).
Tell your doctor if:
- you are not feeling well or find any side effect too uncomfortable or unacceptable
- any side effect becomes worse.
Tell your doctor immediately if any of the following conditions occur (because you may be told to stop using Vagifem® Low):
- severe pain or swelling in your legs or sudden chest pain and difficulty breathing
- yellow colouring of the skin and eyes (jaundice) or other liver problems
- migraine-like headache, and you have not previously had migraines
- rise in blood pressure
- you know or suspect you are pregnant.
Cancer of the breast, ovaries or the lining of the womb, blood clots and stroke have been reported with some types of systemic hormone replacement therapy (“systemic” means to affect the body as a whole).
The following additional side effects have been reported to be associated with other types of estrogen treatment:
- heart attack or heart disease
- gall bladder disease
- various skin diseases and itching
- increase in size of uterine fibroids
- epilepsy
- libido disorder
- asthma
- probable dementia.
Do not be alarmed by this list of possible side effects. You may not experience any of them.
Storage
Keep all medicines out of reach of children.
Vagifem® Low should be kept in a cool dry place where the temperature stays below 25°C. Do not put Vagifem® Low in the refrigerator. Keep Vagifem® Low in the outer carton in order to protect from light.
Product Description
What Vagifem® Low looks like
Each Vagifem® Low modified release pessary is inset in a single use, disposable applicator, packed in a blister pack. The modified release pessaries are white and round and marked on one side with ‘Novo 278.’
Vagifem® Low is supplied in:
- 18 packs - 3 blister packs each containing 6 applicators with inset modified release pessaries.
Ingredients
Each modified release pessary contains estradiol hemihydrate equivalent to 10µg estradiol as the active ingredient.
The tablets also contain hypromellose, lactose, maize starch, magnesium stearate, and macrogol 6000.
Manufacturer
Vagifem® Low is supplied in Australia by:
Novo Nordisk Pharmaceuticals Pty Ltd
Level 10, 118 Mount Street
North Sydney NSW 2060
Australia
Australian Registration Number:
AUST R 163054
Vagifem® is a registered trademark of Novo Nordisk Healthcare AG.
© 2023
Novo Nordisk A/S
Always check the following websites to ensure you are reading the most recent version of the Estrofem® consumer medicine information:
www.novonordisk.com.au
https://www.ebs.tga.gov.au/
For further information call Novo Nordisk Customer Care on 1800 668 626.
This leaflet was prepared in October 2023
Directions for Use
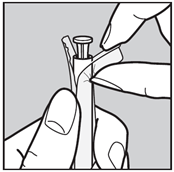
- Wash hands well. Tear off one single blister pack. Open the end as shown in the picture.
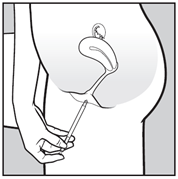
- Insert the applicator carefully into the vagina. Stop when you can feel some resistance (8-10 cm).
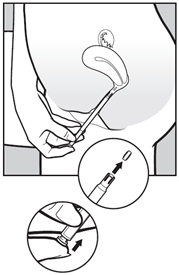
- To release the pessary, gently press the push button until you feel a click. The pessary will stick to the wall of the vagina straight away. It will not fall out if you stand up or walk.
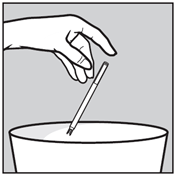
- Take out the applicator and throw it away.
Published by MIMS January 2024

