1 Name of Medicine
Venetoclax.
2 Qualitative and Quantitative Composition
Venclexta 10 mg tablets: each film-coated tablet contains 10 mg venetoclax.
Venclexta 50 mg tablets: each film-coated tablet contains 50 mg venetoclax.
Venclexta 100 mg tablets: each film-coated tablet contains 100 mg venetoclax.
For the full list of excipients, see Section 6.1.
3 Pharmaceutical Form
Film-coated tablet.
Venclexta 10 mg tablets: round, biconvex shaped, pale yellow debossed with "V" on one side and "10" on the other side.
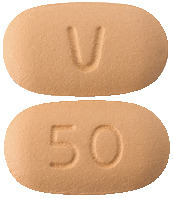
Venclexta 50 mg tablets: oblong, biconvex shaped, beige debossed with "V" on one side and "50" on the other side.
Venclexta 100 mg tablets: oblong, biconvex shaped, pale yellow debossed with "V" on one side and "100" on the other side.
4 Clinical Particulars
4.9 Overdose
Daily doses of up to 1200 mg of Venclexta have been evaluated in clinical trials. There has been no experience with overdose in clinical trials. If an overdose is suspected, treatment should consist of general supportive measures.
For information on the management of overdose in Australia contact the Poison Information Centre on 13 11 26.
5 Pharmacological Properties
5.3 Preclinical Safety Data
Animal pharmacology and/or toxicology. Toxicities observed in animal studies with venetoclax included dose-dependent reductions in lymphocytes and red blood cell mass. After cessation of dosing with venetoclax, red blood cell effects were reversible, whereas partial reversibility of lymphocytes was observed at the end of an 18-week recovery period. Both B- and T-cells were affected, but the most significant decreases occurred with B-cells.
Venetoclax also caused single-cell necrosis in various tissues, including the gallbladder and exocrine pancreas, with no evidence of disruption of tissue integrity or organ dysfunction; these findings were minimal to mild in magnitude. Following a 4-week dosing period and subsequent 4-week recovery period, minimal single-cell necrosis was still present in some tissues and reversibility has not been assessed following longer periods of dosing or recovery.
After approximately 3 months of daily dosing in dogs, venetoclax caused progressive white discoloration of the hair coat, due to loss of melanin pigment in the hair. No changes in the quality of the hair coat or skin were observed, nor in other pigmented tissues examined (e.g. the iris and the ocular fundus of the eye). Reversibility of the hair coat changes has not been assessed in dogs.
The M27 metabolite orally administered to mice had effects similar to those with venetoclax (decreased lymphocytes and red blood cell mass) but of lesser magnitude, consistent with its low pharmacologic activity in vitro.
Genotoxicity. Venetoclax was not mutagenic in an in vitro bacterial mutagenicity (Ames) assay, did not induce numerical or structural aberrations in an in vitro chromosome aberration assay using human peripheral blood lymphocytes, and was not clastogenic in an in vivo mouse bone marrow micronucleus assay at a single oral dose up to 835 mg/kg (~5 times the clinical Cmax at the maximum recommended dose of 600 mg/day). The M27 metabolite was negative for genotoxic activity in in vitro Ames and chromosome aberration assays.
Carcinogenicity. Venetoclax and the M27 major human metabolite were not carcinogenic in a 6-month study in transgenic (Tg.rasH2) mice at oral doses up to 400 mg/kg/day of venetoclax and 250 mg/kg/day of M27. Exposure margins (based on AUC), relative to patients at a recommended clinical dose of 400 mg/day, were approximately 2-fold for venetoclax and 5.8-fold for M27.
6 Pharmaceutical Particulars
6.7 Physicochemical Properties
Venetoclax is described chemically as 4-(4-{[2-(4-chlorophenyl)- 4,4-dimethylcyclohex-1-en-1-yl]methyl}piperazin-1-yl)- N-({3-nitro-4-[(tetrahydro-2H-pyran-4-ylmethyl)amino] phenyl}sulfonyl)-2-(1H-pyrrolo[2,3-b] pyridin-5-yloxy)benzamide.
Chemical structure.
https://stagingapi.mims.com/au/public/v2/images/fullchemgif/CSVENETO.gif Empirical formula: C45H50ClN7O7S.
Molecular weight: 868.44.
CAS number. 1257044-40-8.
7 Medicine Schedule (Poisons Standard)
Schedule 4 - Prescription Only Medicine.
Summary Table of Changes
https://stagingapi.mims.com/au/public/v2/images/fulltablegif/VENCLEST.gif