What is in this leaflet
This leaflet answers some common questions about Vimpat.
It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking Vimpat against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine. You may need to read it again.
What Vimpat is used for
Vimpat tablets and oral solution are used in patients over 4 years in combination with other medicines to control epilepsy. Vimpat tablets and oral solution can only be used by itself in patients over 16 years. Epilepsy is a condition where you have repeated seizures. There are many different types of seizures, ranging from mild to severe.
This medicine belongs to a group of medicines called antiepileptics. These medicines are thought to work by controlling brain chemicals which send signals to nerves so that seizures do not happen.
Your doctor may have prescribed this medicine for another reason.
Ask your doctor if you have any questions about why this medicine has been prescribed for you.
There is no evidence that Vimpat is addictive.
This medicine is available only with a doctor’s prescription.
Vimpat is not recommended for use in children under the age of 4 years as its safety and effectiveness has not been established in this age group.
Before you take Vimpat
When you must not take it
Do not take Vimpat if you have an allergy to:
- Lacosamide or any of the ingredients listed at the end of this leaflet.
Some of the symptoms of an allergic reaction may include:
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body
- rash, itching or hives on the skin.
Do not take Vimpat if you have, or have had, a heart condition causing an uneven heart beat.
If you are not sure whether any of the above conditions apply to you, ask your doctor.
Do not take this medicine after the expiry date printed on the pack.
Do not take this medicine if the packaging is torn or shows signs of tampering. If it has expired or is damaged, return to your pharmacist for disposal.
If you are not sure whether you should start taking this medicine, talk to your doctor or pharmacist.
Before you start to take it
Tell your doctor or pharmacist if you:
- are taking any other medicines, especially barbiturates (such as phenobarbitone) or any other antiepileptic medicines (such as carbamazepine, lamotrigine or levetiracetam)
- have allergies to any other substances, such as foods, preservatives or dyes.
Tell your doctor if you have, or have had, any medical conditions, especially the following:
- heart problems
- kidney problems
- liver problems
- any mental health condition, such as depression.
Tell your doctor if you are pregnant or intend to become pregnant. Vimpat may affect your developing baby if you take it during pregnancy. However, it is very important to control your seizures while you are pregnant. Your doctor will outline and weigh up all the risks and benefits of taking Vimpat during pregnancy to help decide whether or not you should take it.
Tell your doctor if you are breastfeeding or plan to breastfeed. Your doctor will discuss the risks and benefits of using Vimpat if you are breastfeeding.
If you have not told your doctor or pharmacist about any of the above, tell them before you start taking Vimpat.
Taking other medicines
Tell your doctor or pharmacist if you are taking any other medicines, including any that you buy without a prescription from your pharmacy, supermarket or health food shop.
Some medicines and Vimpat may interfere with each other. These include:
- medicines to treat heart problems
- medicine which may have an affect on your heart beat such as carbamazepine, lamotrigine or pregabalin.
Vimpat does not interact with the oral contraceptive pill.
However, you may be given Vimpat together with other antiepileptic medicines that do interact and may affect the effectiveness of your contraceptive. Your doctor may advise you to use an additional method of contraception if you take Vimpat with other antiepileptic medicines.
How to take Vimpat
How much to take
Follow all directions given to you by your doctor carefully. They may differ from the information contained in this leaflet.
Your doctor will tell you how much Vimpat you will need to take each day. This may depend on your condition, your body weight, and whether or not you are taking any other medicines.
Your doctor may start you on a low dose of Vimpat first of 50 mg or 100 mg twice a day. Your doctor may slowly increase your dose up to a maximum of 300 mg twice a day, until you are taking enough to control your epilepsy and you are not having any seizures.
For use in children weighing less than 50kg, the doctor may start you on a low dose of Vimpat oral solution first of 0.1 mL/kg twice a day. The doctor may slowly increase your dose up to a maximum of 0.6 mL/kg twice a day, until you are taking enough to control your epilepsy and you are not having any seizures.
If you do not understand the instructions on the pack, ask your doctor or pharmacist for help.
How to take it
Tablets
Swallow Vimpat tablets whole with a glass of water.
Oral Solution
Use only the syringe within this pack. If you need to take a dose greater than 10 mL (100 mg), use the syringe a second time to draw up the correct amount of solution. Open the bottle by pressing the cap while turning it anti-clockwise (1).
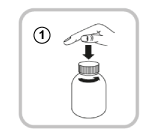
Follow these steps the first time you take Vimpat:
- Take off the adaptor from the oral syringe (2).
- Put the adaptor into the top of the bottle (3). Make sure it is fixed well in place. You do not need to remove the adaptor after use.
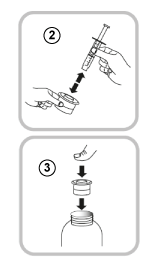
Follow these steps each time you take Vimpat:
- Put the oral syringe into the adaptor opening (4).
- Turn the bottle upside down (5).
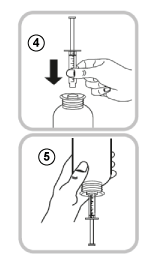
- Hold the bottle upside down in one hand and use the other hand to fill the oral syringe.
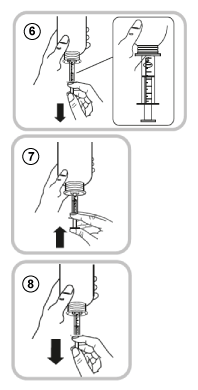
- Pull the piston down to fill the oral syringe with a small amount of solution (6).
- Push the piston up to get rid of any bubbles (7).
- Pull the piston down to the millilitre (mL) dose marker prescribed by your doctor (8).
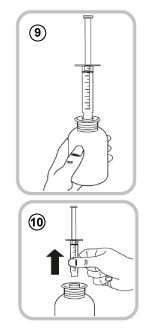
- Turn the bottle the right way up (9).
- Take the oral syringe out of the adaptor (10).
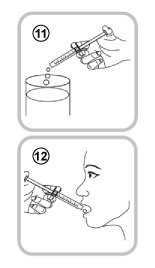
- Empty the contents of the oral syringe into a little water by pushing the piston to the bottom of the oral syringe (11) – you will then need to drink all of the water (add just enough to make it easy to drink) or
- Drink the solution directly from the oral syringe without water (12) – drink the whole contents of the oral syringe.
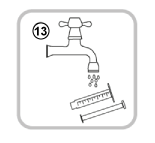
- Close the bottle with the plastic screw cap (you do not need to remove the adaptor).
- Wash the oral syringe with water only (13).
When to take it
Take Vimpat twice a day, once in the morning and once at night. Take it at about the same time each day. Taking your medicine at the same time each day will have the best effect. It will also help you remember when to take it.
It does not matter if you take this medicine before or after food.
If you forget to take it
Contact your doctor if you have missed one or more doses.
Do not take a double dose to make up for the dose that you missed. This may increase the chance of you getting an unwanted side effect.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering to take your medicine, ask your pharmacist for some hints.
How long to take it
Most antiepileptic medicines take time to work, so do not be discouraged if you do not feel better straight away.
Continue taking your medicine for as long as your doctor tells you to. This medicine helps control your condition, but does not cure it. Therefore you must take your medicine every day, even if you feel well.
Do not stop taking Vimpat, or change the dosage, without checking with your doctor. Do not let yourself run out of medicine over the weekend or on holidays. Stopping Vimpat suddenly may cause unwanted side effects or make your condition worse. Your doctor will slowly reduce your dose before you can stop taking it completely.
If you take too much (overdose)
Immediately telephone your doctor or the Poisons Information Centre (telephone in Australia 13 11 26, in New Zealand 0800 POISON or 0800 764 766), or go to Accident and Emergency at your nearest hospital if you think that you or anyone else may have taken too much Vimpat. Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
Symptoms of overdose may include feeling dizzy, drowsy or having an upset stomach.
While you are using Vimpat
Things you must do
Tell your doctor immediately if you notice an increase in seizures.
Tell your doctor immediately if you have symptoms of depression or thoughts of harming yourself.
Tell any other doctors, dentists, and pharmacists who are treating you that you are taking this medicine.
If you are about to be started on any new medicine, tell your doctor, dentist or pharmacist that you are taking Vimpat.
Before you have any surgery or emergency treatment, tell your doctor or dentist that you are taking Vimpat.
Tell your doctor if you feel Vimpat is not helping your condition. Your doctor may need to change your medicine.
Tell your doctor if, for any reason, you have not taken this medicine exactly as prescribed. Otherwise, your doctor may change your treatment unnecessarily.
If you become pregnant while taking this medicine, tell your doctor.
Be sure to keep all of your doctor’s appointments so that your progress can be checked. Your doctor will check your progress and may want to take some tests from time to time. This helps to prevent unwanted side effects.
Things you must not do
Do not give Vimpat to anyone else, even if their symptoms seem similar to yours or they have the same condition as you.
Do not take Vimpat to treat any other complaints unless your doctor tells you to.
Do not stop taking Vimpat or change the dosage unless your doctor tells you to. Stopping Vimpat suddenly may cause unwanted side effects or make your condition worse.
Things to be careful of
Be careful driving or operating machinery until you know how Vimpat affects you.
As with other antiepileptic medicines Vimpat may cause dizziness or drowsiness. This is more frequent at the beginning of treatment or after an increase in the dose.
If you are feeling dizzy or drowsy, do not drive a car, operate machinery, or do anything else that could be dangerous.
As a safety precaution, do not take Vimpat with alcohol.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking Vimpat.
This medicine helps most people with epilepsy but it may have unwanted side effects in a few people. All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical treatment if you get some of the side effects.
Do not be alarmed by this list of side effects. You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
If you get any side effects, do not stop taking Vimpat without first talking to your doctor or pharmacist.
Tell your doctor or pharmacist if you notice any of the following and they worry you:
- dizziness or problems with balance or coordination
- headache
- nausea (feeling sick) or vomiting
- feeling tired, drowsy or sleepy
- forgetfulness
- tremors
- itching.
The above list includes the more common side effects of your medicine. They are mostly mild and short-lived.
Tell your doctor as soon as possible if you notice any of the following:
- feelings of depression
- feeling aggressive or agitated
- spinning sensations
- double vision or blurred vision
- having trouble sleeping.
The above list includes serious side effects that may require medical attention.
If any of the following happen, tell your doctor immediately or go to Accident and Emergency at your nearest hospital:
- thoughts of harming yourself
- more frequent or more severe seizures
- fainting or feeling lightheaded
- heart palpitations or a rapid or irregular pulse
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body
- rash, itching or hives on the skin.
The above list includes more serious side effects. You may need urgent medical attention or hospitalisation.
Tell your doctor if you notice anything else that is making you feel unwell. Other side effects not listed above may happen in some people.
After using Vimpat
Storage
Tablets
Keep your tablets in the pack until it is time to take them. If you take the tablets out of the pack they will not keep well.
Oral Solution
Once you have opened the bottle, you must not use it longer than 2 months.
Keep your tablets and oral solution in a cool dry place where the temperature stays below 30°C
Do not store Vimpat or any other medicine in the bathroom or near a sink.
Do not leave your medicine on a window sill or in the car on hot days. Heat and dampness can destroy some medicines.
Keep Vimpat where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking this medicine or the expiry date has passed, ask your pharmacist what to do with any medicine that is left over.
Product description
What it looks like
Tablets
Vimpat tablets are available in four strengths:
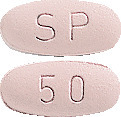
- 50 mg – pinkish, oval tablets with 'SP' stamped on one side and '50' stamped on the other
- 100 mg – dark yellow, oval tablets with 'SP' stamped on one side and '100' stamped on the other
- 150 mg – salmon, oval tablets with 'SP' stamped on one side and '150' stamped on the other
- 200 mg – blue, oval tablets with 'SP' stamped on one side and '200' stamped on the other.
Oral Solution
Vimpat Oral solution is packed in a 200 mL amber glass bottle and is available as 10 mg/mL strength. The pack also contains an oral syringe.
Ingredients
Tablets
Each Vimpat tablet contains either 50 mg, 100 mg, 150 mg or 200 mg of lacosamide as the active ingredient.
Other ingredients in Vimpat tablets include:
- colloidal anhydrous silica
- crospovidone
- hydroxypropylcellulose
- magnesium stearate
- microcrystalline cellulose
- macrogol 3350
- polyvinyl alcohol
- purified talc
- titanium dioxide.
The following strengths also contain:
- 50 mg - iron oxide red C177491, iron oxide black C177499, indigo carmine C173015
- 100 mg - iron oxide yellow C177492
- 150 mg -iron oxide yellow C177492, iron oxide red C177491, iron oxide black C177499
- 200 mg - indigo carmine C173015.
Vimpat tablets do not contain lactose, sucrose, gluten, tartrazine or any other azo dyes.
Oral Solution
The oral solution contains 10 mg of lacosamide per millilitre and the following ingredients: glycerol, carmellose sodium, sorbitol solution (70 per cent) (crystallising), macrogol 4000, sodium chloride, citric acid, acesulfame potassium, sodium methyl hydroxybenzoate, Strawberry flavor 501440 T, Masking flavor 501521 T, water- purified.
Australian Sponsor:
UCB Pharma
A division of UCB Australia Pty Ltd
Level 1, 1155 Malvern Road
Malvern Vic 3144,
Australia
NZ Sponsor:
Seqirus (NZ) Ltd
PO Box 62 590
Greenlane
Auckland 1546
Phone: 0800 502 757
Vimpat 50 mg tablets
- AUST R 196449
Vimpat 100 mg tablets
- AUST R 196450
Vimpat 150 mg tablets
- AUST R 196451
Vimpat 200 mg tablets
- AUST R 196452
Vimpat 10 mg/mL oral solution
- AUST R 286810
Date of preparation:
March 2021
VIMPAT is a registered trademark used by UCB Pharma GmbH under license.
Published by MIMS May 2021