What is in this leaflet
This leaflet answers some common questions about Xolair.
It does not contain all the available information. It does not take the place of talking to your doctor, pharmacist or healthcare provider.
The information in this leaflet was last updated on the date listed on the final page. More recent information on the medicine may be available.
You should ensure that you speak to your pharmacist or doctor to obtain the most up to date information on the medicine. You can also download the most up to date leaflet from www.novartis.com.au. Those updates may contain important information about the medicine and its use of which you should be aware.
All medicines have risks and benefits. Your doctor has weighed the risks of you having Xolair against the benefits they expect it will have for you.
If you have any concerns about having this medicine, ask your doctor or pharmacist.
Keep this leaflet. You may need to read it again.
What Xolair is used for
Allergic asthma
Xolair is used to prevent or relieve the symptoms of allergic asthma in people who are already using preventer puffers containing steroids. Asthma is a disease where the lining of the lungs becomes inflamed (red and swollen), making it difficult to breathe. This may be due to a reaction to house dust mites, smoke or other irritants.
This medicine can be used in adults and in children aged 6 years or over in allergic asthma.
Chronic Rhinosinusitis with Nasal Polyps (Nasal Polyps)
Xolair is used to treat nasal polyps in adults (18 years of age and older) whose severe disease is not well controlled with their current nasal polyps medicines. Xolair helps reduce the size of the polyps and improves symptoms caused by nasal polyps including nasal congestion, loss of sense of smell, post-nasal drip and runny nose.
This medicine can be used in adults aged 18 years and over in nasal polyps. Its use in children and adolescents below 18 years of age with nasal polyps has not been studied.
How Xolair Works
Xolair contains omalizumab, a monoclonal antibody produced by recombinant DNA technology. It works by blocking a substance produced by the body called immunoglobulin E (or IgE). IgE contributes to a type of inflammation that is involved in causing symptoms of asthma and nasal polyps. Your doctor will measure the amount of IgE in your blood before starting your treatment with Xolair.
Chronic Spontaneous Urticaria (CSU)
Xolair is used to treat Chronic Spontaneous Urticaria in adults and adolescents (12 years of age and older) whose CSU symptoms are not well controlled by antihistamines.
Xolair works by blocking a substance called immunoglobulin E (also known as IgE) which is produced by your body. As a consequence the activity of specific receptors and/or cells in your body which play a key role in causing chronic spontaneous urticaria are reduced. This leads to a reduction of your symptoms, like itching and hives.
Elderly patients are given the same dosage as younger adults.
Ask your doctor if you have any questions about why Xolair has been prescribed for you. Your doctor may have prescribed this medicine for another reason.
Xolair is only available with a doctor's prescription. It is not addictive.
Before you have Xolair
When you must not have it
Do not have Xolair if you have an allergy to:
- omalizumab (the active ingredient in Xolair) or any of the other ingredients listed at the end of this leaflet.
Symptoms of an allergic reaction may include:
- wheezing or shortness of breath
- severe rash, itching or hives on the skin
- swelling of the face, lips, tongue, larynx (voice box), windpipe which may cause difficulty in swallowing or breathing, or other parts of the body
- fast heartbeat, dizziness and light headedness
Before you have it
Tell your doctor if you get severe allergic reactions (called anaphylaxis) to any medicines, foods, dyes, preservatives, bee stings, insect bites, latex etc.
Tell your doctor if you have any of the following conditions:
- kidney or liver problems
- autoimmune disease
- If you live in a region where parasite infections are frequent, Xolair may weaken your resistance to such infections.
- If you have had a previous severe allergic reaction (anaphylaxis) resulting, for example, from medicine, an insect bite or food.
- If you had an allergic reaction to latex. The needle cap of the pre-filled syringe may contain dry rubber (latex).
- a low platelet count (thrombocytopenia)
You should not use Xolair to prevent or treat other allergy-type conditions:
- sudden allergic reactions
- hyperimmunoglobulin E syndrome (an inherited immunodeficient disorder)
- aspergillosis (a fungus-related lung disease)
- food allergy, allergic skin rash or hay fever.
You should not use Xolair to treat acute asthma symptoms, like a sudden asthma attack. You will have been given a separate medicine for this.
Tell your doctor if you are pregnant or intend to become pregnant or wish to breast-feed your baby. There is not much information on the use of Xolair during pregnancy or breast-feeding. If it is necessary for you to have this medicine, your doctor will discuss with you the benefits and risks involved.
If you have not told your doctor about any of these things, tell him/her before you have Xolair.
Look out for signs of allergic reactions and other serious side effects. This medicine can cause such reactions.
It is important that you receive training from your doctor or healthcare professional on how to recognise early signs of severe allergic reactions, and how to manage these reactions if they occur – see "Side effects". The majority of severe allergic reactions occur within the first 3 doses, and within the first hour of taking Xolair.
Taking other medicines
Tell your doctor if you are taking any other medicines, including any that you buy without a prescription from a pharmacy, supermarket or health food shop.
Xolair has been used together with inhaled and/or intranasal corticosteroids other common medicines for asthma/or nasal polyps, as well as H1 or H2 antihistamines and LTRAs for chronic spontaneous urticaria. It is possible that some medicines and Xolair could interfere with each other. Your doctor or pharmacist can advise you.
How Xolair is given
Follow all directions given to you by your doctor or healthcare professional carefully.
Xolair is given as a subcutaneous injection. This means that it is injected into the fatty tissue just under the skin.
There are two different ways Xolair can be given:
- Pre-filled syringe: designed to be administered by patients or carers or healthcare professionals.
- Powder for injection vial with diluent: for use by healthcare professionals.
The injections are usually given in the upper arm or thigh.
Injecting Xolair
You and your doctor will decide if you should inject Xolair yourself. The first 3 doses is recommended to be given by or under the supervision of a healthcare professional.
For detailed instructions on how to inject Xolair pre-filled syringe, see "Instructions for use" at the end of the leaflet.
How much Xolair to use
Allergic asthma and Chronic Rhinosinusitis with Nasal Polyps
Your doctor will decide how much Xolair you need and how often you will need it. This depends on your body weight and the amount of IgE measured in your blood before the start of treatment.
You will need 1 to 4 injections at a time. The recommended dose of Xolair ranges from 75 to 750 mg of Xolair either every two weeks or every four weeks as prescribed by your doctor.
Keep taking your current asthma and/or nasal polyps medicine during Xolair treatment.
Do not stop taking any asthma and/or nasal polyps medications without talking to your doctor. However after 16 weeks, if your symptoms are well controlled, your doctor may be able to reduce or stop other asthma and/or nasal polyps medicines that you are taking. Your doctor will discuss this with you.
Chronic spontaneous urticaria
You will need 1 or 2 injections of 150 mg at a time every four weeks.
Keep taking your current medicine for CSU during Xolair treatment.
Do not stop taking any medicine without talking to your doctor. However, if you are being treated for CSU, your doctor may stop Xolair treatment from time to time so that your symptoms can be assessed. Follow your doctor's instructions.
If you forget to use it
If you have missed an appointment to get a Xolair injection, contact your doctor and get it as soon as you remember.
If you have forgotten a dose of Xolair, inject the dose as soon as you remember. Then talk to your doctor to discuss when to inject the next dose.
If you use too much (Overdose)
If you accidently use more Xolair than prescribed, immediately tell your doctor for further advice.
No cases of overdose with this medicine have been reported.
While you are having Xolair
Things you must do
If you become pregnant while having treatment with Xolair, tell your doctor.
Keep all of your doctor's appointments so that you don't miss any injections and your progress can be checked.
If you are about to be started on any new medicine, remind your doctor and pharmacist that you are having Xolair.
Tell any other doctor, dentist or pharmacist who treats you that you are having Xolair.
Things to be careful of
Be careful driving, operating machinery or doing jobs that require you to be alert until you know how Xolair affects you.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are having Xolair.
All medicines can have side effects. Sometimes they are serious, but most of the time they are not. You may need medical treatment if you get some of the side effects.
Do not be alarmed by this list of possible side effects. You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor if you notice any of the following side effects and they worry you:
- bruising, redness or pain at the injection site
- mild skin rash
- headache
- tiredness
- hair loss
- joint swelling
- fever (very common in children)
- sore throat and blocked nose (nasopharyngitis)
- feeling of pressure or pain in the cheeks and forehead (sinusitis and sinus headache)
- upper respiratory tract infection, such as inflammation of the pharynx and common cold
- burning sensation or pain when passing urine and having to urinate frequently (possible symptom of an urinary tract infection)
- pain in upper or lower limbs (arms and/or legs)
- pain in the upper part of the abdomen
- pain in muscles and/or bones and/or joints (musculoskeletal pain, myalgia, arthralgia)
Tell your doctor immediately if you notice any of the following signs:
- abnormal bleeding or bruising
- pain, numbness or tingling in the arms and legs, lumps or raised patches in the skin, weakness and fatigue, loss of appetite, and weight loss
Tell your doctor immediately or go to Accident and Emergency at your nearest hospital if you notice any signs of an allergic reaction to Xolair.
Xolair contains a protein, and proteins can cause serious allergic reactions in some people.
Common symptoms of an allergic reaction may include:
- wheezing, chest tightness and cough
- a raised, itchy, red rash (hives)
- swollen lips, tongue, eyes or face
- stomach pain, feeling sick, vomiting or diarrhoea
Sometimes patients experience a severe allergic reaction. Anaphylaxis is one type of severe allergic reaction which can affect multiple parts of the body. Rare cases of anaphylaxis have been reported with Xolair.
Telephone 000 immediately if severe symptoms of allergic reaction (anaphylaxis) occur.
Symptoms of severe allergic reactions (including anaphylaxis) include any of the above symptoms, plus:
- swelling of the throat and mouth
- difficulty breathing
- lightheadedness
- confusion
- blue skin or lips
- collapsing and losing consciousness
A specific type of allergic reaction (serum sickness) has also been observed in patients treated with Xolair or similar products. Signs include joint pain, stiffness, rash, fever, swollen/enlarged lymph nodes and occur typically within one to five days after the injection.
Churg-Strauss syndrome have been observed in patients treated with Xolair. The symptoms may include one or more of the following: pain, numbness or tingling in the arm and legs, lumps or raised patches in the skin, weakness and fatigue, loss of appetite and weight loss.
Tell your doctor if you notice anything else that is making you feel unwell. Other side effects not listed above may happen in some people.
After using Xolair
Storage
- Keep the pre-filled syringes in the original container until it is time for use.
- Keep them in the refrigerator. Do not freeze them.
- Do not store Xolair or any other medicine in the bathroom or near a sink.
- Do not leave them in the car or on window sills.
- Do not use Xolair beyond the expiry date on the label.
- Do not use the product if there are signs of deterioration.
- Do not use Xolair if the pack is damaged or shows signs of tampering.
Heat and dampness can destroy some medicines. Xolair will keep well if it is kept cool and dry.
Keep the medicine where children cannot reach it.
Disposal
If your doctor stops your treatment with Xolair or you find that it has passed the expiry date, ask your pharmacist how to dispose of medicines you no longer use.
Product description
What it looks like
Solution for injection in pre-filled syringe:
Each pack of Xolair contains 1 pre-filled syringe. One syringe of 0.5 ml solution contains 75 mg omalizumab. One syringe of 1 ml solution contains 150 mg omalizumab.
Vial powder for injection:
Each pack of Xolair contains a 150 mg vial of sterile powder and one ampoule of sterile water for injections for use in dissolving the powder.
Ingredients
Solution for injection in pre-filled syringe:
Each pre-filled syringe of Xolair contains the active ingredient, omalizumab. It also contains:
- L- arginine hydrochloride
- L-histidine
- L-histidine hydrochloride monohydrate
- polysorbate 20
- water for injections
Vial powder for injection:
Each vial of Xolair contains omalizumab. It also contains:
- Sucrose
- L-histidine
- L-histidine hydrochloride monohydrate
- polysorbate 20
Sponsor
Xolair is supplied in Australia by:
NOVARTIS Pharmaceuticals Australia Pty Limited
ABN 18 004 244 160
54 Waterloo Road
Macquarie Park NSW 2113
Telephone 1-800-671-203
®= Registered Trademark
This leaflet was prepared in June 2023.
Australian Registration Numbers
Xolair 150 mg solution for injection in pre-filled syringe AUST R 201126
Xolair 75 mg solution for injection in pre-filled syringe AUST R 201124
Xolair 150 mg vial powder for injection with diluent AUST R 82744
(xol010623c.doc) based on PI (xol010623i.doc)
INSTRUCTIONS FOR USE
XOLAIR pre-filled syringe
omalizumab
Read ALL the way through these instructions before injecting. If your doctor decides that you or a caregiver may be able to give your injections of Xolair at home, you need to be trained by your doctor, nurse or pharmacist before you inject yourself or others. Children (6 to less than 12 years of age) are not expected to inject Xolair themselves, however, if deemed appropriate by their doctor, a caregiver may give them their Xolair injection after proper training. The box contains a Xolair pre-filled syringe individually sealed in a plastic tray.
Xolair pre-filled syringe is available in two strengths, 75 mg and 150 mg. You may receive one or both strengths from the pharmacy.
Your Xolair 75 mg pre-filled syringe
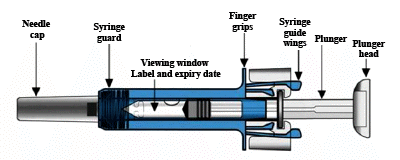
Your Xolair 150 mg pre-filled syringe
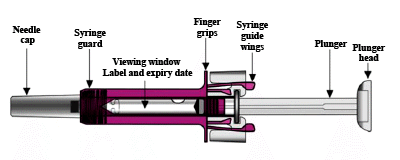
After the medicine has been injected the syringe guard will be activated to cover the needle. This is intended to protect against accidental needle stick injuries.
Other items you need for your injection:
- Alcohol swab.
- Cotton ball or gauze.
- Sharps disposal container.
- Adhesive plaster

Important safety information
Caution: Keep the syringe out of the sight and reach of children.
- The needle cap of the syringe may contain dry rubber (latex), which should not be handled by anyone who is sensitive to this substance.
- Do not open the sealed outer box until you are ready to use this medicine.
- Do not use this medicine if either the seal on the outer box or the seal of the plastic tray is broken, as it may not be safe for you to use.
- Do not use if the syringe has been dropped onto a hard surface or dropped after removing the needle cap.
- Never leave the syringe where others might tamper with it.
- Do not shake the syringe.
- Be careful not to touch the syringe guard wings before use. If the wings are touched, the syringe guard may be activated too early.
- Do not remove the needle cap until just before you give the injection.
- The syringe cannot be re-used. Dispose of the used syringe immediately after use in a sharps container.
Storage of the Xolair pre-filled syringe
- Store this medicine sealed in its outer box to protect it from light. Store in the refrigerator between 2°C and 8°C. DO NOT FREEZE.
- Prior to use, allow the syringe to reach room temperature (25°C) before preparing it for injection (it will take about 30 minutes). If necessary, the product may be returned to the refrigerator for later use. The total time that the syringe is kept at room temperature must not exceed 48 hours.
- DO NOT try to warm the syringe using an external heat source.
- Do not use the syringe after the expiry date which is stated on the outer box and syringe label. If it has expired, return the entire pack to the pharmacy.
The injection site
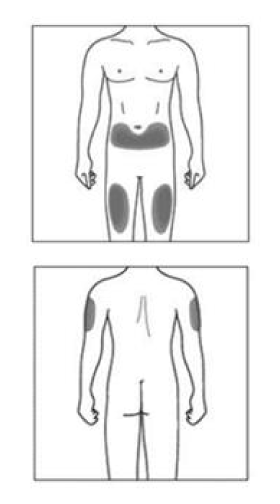
The injection site is the place on the body where you are going to use the syringe.
- The recommended site is the front of the thighs. You may also use the lower abdomen, but NOT the area 5 centimeters around the navel (belly button).
- If you need to give more than one injection for the full dose, choose a different injection site each time you inject.
- If injecting more than once into the same area, ensure a distance of at least 4 cm between injection sites.
- Do not inject into areas where the skin is tender, bruised, red, or hard. Avoid areas with scars or stretch marks.
If a caregiver is giving the injection, the outer upper arms may also be used.
Preparing the Xolair pre-filled syringe for use
Note: Depending on the dose prescribed to you by your doctor, you may need to prepare one or more pre-filled syringes, and inject the contents of them all.
- Take the box containing the syringe out of the refrigerator and leave it UNOPENED for about 20 minutes so that it reaches room temperature (leave the syringe in the box to protect it from light).
- When you are ready to use the syringe, wash your hands thoroughly with soap and water.
- Clean the injection site with an alcohol swab.
- Remove the plastic tray from the box and peel back the paper cover. Gripping the middle of the syringe guard, lift the syringe out of the tray.
- Inspect the syringe. The liquid should be clear to slightly cloudy. Its color may vary from colorless to pale brownish-yellow. You may see an air bubble, which is normal. DO NOT USE if the syringe is broken or if the liquid looks distinctly cloudy or distinctly brown, or contains particles. In all these cases, return the entire pack to the pharmacy.
- Holding the syringe horizontally, look into the viewing window to check the expiry date printed on the label. (Note: It is possible to rotate the inner part of the syringe assembly so that the label can be read in the viewing window. DO NOT USE if the product has expired. If expired, return the entire pack to the pharmacy.)
How to use the Xolair pre-filled syringe
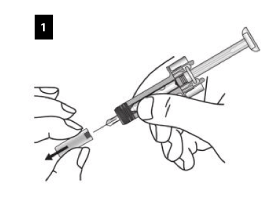
Carefully remove the needle cap from the syringe. Discard the needle cap. You may see a drop of liquid at the end of the needle. This is normal.
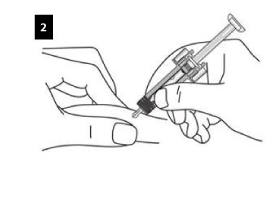
Gently pinch the skin at the injection site and insert the needle as shown. Push the needle all the way in to ensure that the medicine can be fully administered.
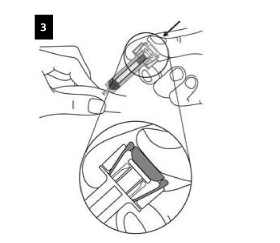
Hold the syringe as shown. SLOWLY depress the plunger AS FAR AS IT WILL GO so that the plunger head is completely between the syringe guard wings.
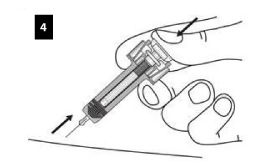
KEEP THE PLUNGER FULLY DEPRESSED while you carefully lift the needle straight out from the injection site.
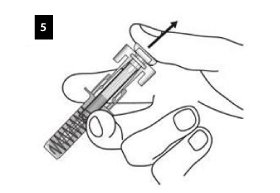
Slowly release the plunger and allow the syringe guard to automatically cover the exposed needle.
There may be a small amount of blood at the injection site. You can press a cotton ball or gauze over the injection site and hold it for 30 seconds. Do not rub the injection site. You may cover the injection site with a small adhesive bandage, if needed.
Disposal instructions
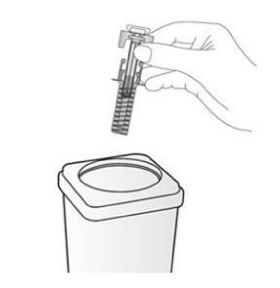
Dispose of the used syringe immediately in a sharps container (closable, puncture resistant container). For the safety and health of you and others, needles and used syringes MUST NEVER be re-used. Any unused medicinal product or waste material should be disposed of in accordance with local requirements. Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
Published by MIMS July 2023
