What is in this leaflet
Read this leaflet carefully before taking your medicine.
This leaflet answers some common questions about ZETIN. It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
The information in this leaflet was last updated on the date listed on the last page. More recent information on this medicine may be available.
Ask your doctor or pharmacist:
- if there is anything you do not understand in this leaflet,
- if you are worried about taking your medicine, or
- to obtain the most up-to-date information.
All medicines have risks and benefits. Your doctor has weighed the risks of you using this medicine against the benefits they expect it will have for you.
Pharmaceutical companies cannot give you medical advice or an individual diagnosis.
Keep this leaflet with your medicine. You may want to read it again.
What this medicine is used for
The name of your medicine is ZETIN. It contains the active ingredient acitretin.
It is used to treat:
- psoriasis
- keratinisation disorders
Ask your doctor if you have any questions about why this medicine has been prescribed for you. Your doctor may have prescribed this medicine for another reason.
This medicine is available only with a doctor's prescription.
How it works
Acitretin, the active ingredient of ZETIN, belongs to a group of medicines called retinoids.
It is very similar to Vitamin A which is obtained from our diet and is vital for the normal growth and development of the body, especially the skin.
Psoriasis is a skin disease with thickened patches of red skin, often with silvery scales. ZETIN is used to treat severe psoriasis and other severe skin disorders.
ZETIN works to return skin to normal when problems with the normal development of the skin are present, as in the case of severe psoriasis and some other skin disorders.
There is no evidence that this medicine is addictive.
Use in children
ZETIN capsules should only be taken by children where alternative therapy cannot be used.
Before you take this medicine
When you must not take it
Do not take this medicine if:
-
You have or have had any of the following:
- severe kidney disease
- severe liver disease
- abnormally high levels of fat in your blood
- an allergic reaction to retinoids or Vitamin A -
You are pregnant or you intend to become pregnant in the next 36 months.
Any possibility that you may be pregnant must be ruled out by both yourself and your doctor before you start taking ZETIN capsules. The result of a pregnancy test must be negative when performed within two weeks before beginning of ZETIN treatment. ZETIN is highly teratogenic, i.e. there is an extremely high risk of having a baby that is severely deformed.
This means you must use effective contraception (preferably 2 complementary methods) for one month before, during and 3 years after treatment with ZETIN.
You must also tell your doctor immediately if you become pregnant in the 3 years following the end of treatment. -
You are breastfeeding.
Breastfeeding must stop before ZETIN treatment can start. Do not breastfeed while taking ZETIN capsules. - You are taking tetracycline antibiotics. These include doxycycline HCl, Doxine®, Doxy®, Achromycin® and Minomycin®.
- You are taking vitamin A, or preparations that contain vitamin A.
- You are taking methotrexate.
-
You are hypersensitive to, or have had an allergic reaction to, acitretin or any of the ingredients listed at the end of this leaflet.
Symptoms of an allergic reaction may include: cough, shortness of breath, wheezing or difficulty breathing; swelling of the face, lips, tongue, throat or other parts of the body; rash, itching or hives on the skin; fainting; or hay fever-like symptoms.
If you think you are having an allergic reaction, do not take any more of the medicine and contact your doctor immediately or go to the Accident and Emergency department at the nearest hospital. - The expiry date (EXP) printed on the pack has passed.
- The packaging is torn, shows signs of tampering or it does not look quite right.
Donation of blood by a patient being treated with ZETIN is prohibited during and for three years after completion of treatment with ZETIN.
Before you start to take it
Before you start taking this medicine, tell your doctor if:
- You have allergies to:
- any other medicines
- any other substances, such as foods, preservatives or dyes.
- You have or have had any medical conditions, especially the following:
- diabetes, or family members with diabetes
- liver disease
- high triglycerides or cholesterol levels in the blood, or family members with a history of high blood triglycerides or cholesterol levels
- You are currently pregnant or you plan to become pregnant. Do not take this medicine whilst pregnant.
- You are currently breastfeeding or you plan to breast-feed. Do not take this medicine whilst breastfeeding.
- You are planning to have surgery.
- You are currently receiving or are planning to receive dental treatment.
- You are taking or are planning to take any other medicines. This includes vitamins and supplements that are available from your pharmacy, supermarket or health food shop.
Some medicines may interact with ZETIN. These include:
- tetracycline antibiotics (such as Vibramycin®, Doxine®, Doxy®, Achromycin® and Minomycin®)
- phenytoin
- methotrexate
- alcohol containing medicines
- the "mini-pill", a low-dose progestogen oral contraceptive
- vitamin A, or formulations that contain vitamin A
If you are taking any of these you may need a different dose or you may need to take different medicines.
Other medicines not listed above may also interact with ZETIN.
How to take this medicine
Carefully follow all directions given to you by your doctor. Their instructions may be different to the information in this leaflet.
How much to take
Your doctor will tell you how much of this medicine you should take. This will depend on your condition and whether you are taking any other medicines. This may also consider your bodyweight and whether you develop any side effects.
The starting dose is usually either 25 mg (1 x 25 mg capsule) or 30 mg (3 x 10 mg capsules) per day for 2 to 4 weeks.
Your dose will probably then be adjusted by your doctor when it is known how you respond to ZETIN capsules.
The initial signs of improvement may be seen in the first week but, more often, after 2 or 3 weeks. It may take 2 to 3 months until the full effect is seen.
Do not change your dosage without first checking with your doctor.
Affected skin areas will either peel off or steadily clear. Sometimes more redness or itching may be present at first, but this will normally improve as treatment continues.
How to take it
ZETIN capsules should be swallowed whole with a glass of water or milk.
When to take it
Take this medicine at the same time each day. Taking it at the same time each day will have the best effect and will also help you remember when to take it.
Female patients should wait until the 2nd or 3rd day of their menstrual period before starting ZETIN capsules. This helps to ensure that you are not pregnant before you start to take ZETIN therapy.
How long to take it for
Continue taking your medicine for as long as your doctor tells you.
A temporary increase in psoriasis is sometimes seen when first starting treatment.
Make sure you have enough to last over weekends and holidays.
If you forget to take it
If it is almost time to take your next dose, skip the missed dose and take your next dose at the usual time. Otherwise, take it as soon as you remember and then go back to taking your medicine as you would normally.
Do not take a double dose to make up for missed doses. This may increase the chance of you experiencing side effects.
If you have trouble remembering to take your medicine, ask your pharmacist for some hints to help you remember.
If you take too much (overdose)
If you think that you or anyone else may have taken too much of this medicine, immediately telephone your doctor or the Poisons Information Centre (Tel: 13 11 26 in Australia) for advice. Alternatively, go to the Accident and Emergency department at your nearest hospital.
Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
While you are taking this medicine
Things you must do
Tell your doctor that you are taking this medicine if:
- you are about to be started on any new medicine
- you are about to have any blood tests
- you are going to have surgery or an anaesthetic or are going into hospital.
It is critical to keep all of your appointments with your doctor so your progress with ZETIN therapy can be monitored regularly. Your doctor may ask you to do some blood, liver function and other tests from time to time in order to check your progress and pick up any unwanted side effects.
Inform your doctor if, for any reason, you have not taken this medicine exactly as prescribed. Otherwise, your doctor may think this medicine was not effective and change your treatment unnecessarily.
Tell any other doctors, dentists and pharmacists who are treating you that you take this medicine.
Things you must not do
Do not:
- Give this medicine to anyone else, even if their symptoms seem similar to yours.
- Take your medicine to treat any other condition unless your doctor tells you to.
- Stop taking your medicine, or change the dosage, without first checking with your doctor.
Things to be careful of
Decreased night vision has been reported with acitretin therapy. Be careful when driving or operating any vehicle at night.
Possible side effects
Tell your doctor as soon as possible if you do not feel well while you are taking ZETIN or if you have any questions or concerns.
Do not be alarmed by the following lists of side effects. You may not experience any of them. All medicines can have side effects. Sometimes they are serious but most of the time they are not.
Common side effects can include:
- dryness of the lips, mouth, nose, eyes and skin.
Either a moisturiser or petroleum jelly can be used to soften the lining of the nose, lips and the skin - drying and inflammation of mucous membranes
- thirst
- flushing
- difficulty producing tears
- intolerant of contact lenses
- itchiness, redness or rashes
- thinning or peeling of the skin that was not previously affected
- nail fragility or conditions
- eye infections
- abnormal liver tests
- increase in blood cholesterol
- muscle, joint or bone pain
Tell your doctor if you notice any of the following and they worry you:
- headache
- tiredness
- heartburn
- strange hair texture or loss
- more prone to sun burn
- impaired night vision
- noises or ringing in ears
- changes in taste
- hay fever-like symptoms
- nose bleeds
- swelling in limbs
Tell your doctor as soon as possible if you notice any of the following.
These may be serious side effects and you may need medical attention:
- changes in mood including depression or aggression
- male breast enlargement
- changes in hearing
If you experience any of the following, stop taking your medicine and contact your doctor immediately or go to the Accident and Emergency department at your nearest hospital.
These are very serious side effects and you may need urgent medical attention or hospitalisation:
- headache, nausea and vomiting, and visual disturbances can be signs of papilloedema and needs medical investigation
- itchy, yellowing skin, lighter coloured stool or darker urine can be signs of jaundice.
Other side effects not listed above may occur in some patients.
Allergic reactions
If you think you are having an allergic reaction to ZETIN, do not take any more of this medicine and tell your doctor immediately or go to the Accident and Emergency department at your nearest hospital.
Symptoms of an allergic reaction may include some or all of the following:
- cough, shortness of breath, wheezing or difficulty breathing
- swelling of the face, lips, tongue, throat or other parts of the body
- rash, itching or hives on the skin
- fainting
- hay fever-like symptoms.
Storage and disposal
Storage
Keep your medicine in its original packaging until it is time to take it. If you take your medicine out of its original packaging it may not keep well.
Keep your medicine in a cool dry place where the temperature will stay below 25°C.
Do not store your medicine, or any other medicine, in the bathroom or near a sink. Do not leave it on a window sill or in the car. Heat and dampness can destroy some medicines.
Keep this medicine where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking this medicine or it has passed its expiry date, your pharmacist can dispose of the remaining medicine safely.
Product description
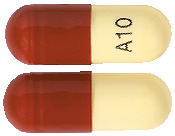
What ZETIN capsules looks like
10 mg Capsule*:
Hard gelatin capsule containing a yellow powder with a white to off-white body and a brown cap printed in black with "A10" on the capsule body.
25 mg Capsule*:
Hard gelatin capsule containing a yellow powder with a yellow to light yellow body and a brown cap printed in black with "A25" on the capsule body.
Available in blister packs of 60 and 100 capsules
* Not all strengths, pack types and/or pack sizes may be available.
Ingredients
Each ZETIN capsule contains 10 or 25 mg of acitretin as the active ingredient.
It also contains the following inactive ingredients:
- maltodextrin
- sodium ascorbate
- microcrystalline cellulose
- gelatin
- sodium lauryl sulphate
- purified water
- black printing ink (shellac glaze, iron oxide black (E172), propylene glycol (E1520))
- iron oxide red (E172) (colourant)
- iron oxide yellow (E172) (colourant) (25mg only)
- titanium dioxide
This medicine is gluten-free, lactose-free, sucrose-free, tartrazine-free and free of other azo dyes.
May contain trace amounts of phenylalanine and sulfites.
Australian Registration Numbers
ZETIN 10 mg capsule (blister): AUST R 196005
ZETIN 25 mg capsule (blister): AUST R 196004
Sponsor
Douglas Pharmaceuticals Australia Pty Ltd
15-17 Chapel Street
Cremorne VIC 3121
Distributed by Arrow Pharmaceuticals Pty Ltd on behalf of
Oraderm Pharmaceuticals Pty Ltd
15-17 Chapel Street
Cremorne VIC 3121
This leaflet was last updated in September 2021.
Published by MIMS October 2021